Abstract
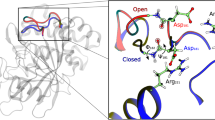
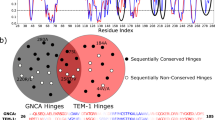
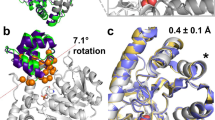
In the early 2000s, Tawfik presented his ‘New View’ on enzyme evolution, highlighting the role of conformational plasticity in expanding the functional diversity of limited repertoires of sequences. This view is gaining increasing traction with increasing evidence of the importance of conformational dynamics in both natural and laboratory evolution of enzymes. The past years have seen several elegant examples of harnessing conformational (particularly loop) dynamics to successfully manipulate protein function. This Review revisits flexible loops as critical participants in regulating enzyme activity. We showcase several systems of particular interest: triosephosphate isomerase barrel proteins, protein tyrosine phosphatases and β-lactamases, while briefly discussing other systems in which loop dynamics are important for selectivity and turnover. We then discuss the implications for engineering, presenting examples of successful loop manipulation in either improving catalytic efficiency, or changing selectivity completely. Overall, it is becoming clearer that mimicking nature by manipulating the conformational dynamics of key protein loops is a powerful method of tailoring enzyme activity, without needing to target active-site residues.

This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
Change history
20 June 2023
A Correction to this paper has been published: https://doi.org/10.1038/s41570-023-00515-9
References
James, L. C. & Tawfik, D. S. Conformational diversity and protein evolution — a 60-year-old hypothesis revisited. Trends Biochem. Sci. 28, P361–P368 (2003).
Tokuriki, N. & Tawfik, D. S. Protein dynamism and evolvability. Science 324, 203–207 (2009).
Damry, A. M. & Jackson, C. J. The evolution and engineering of enzyme activity through tuning conformational landscapes. Prot. Eng. Des. Sel. 34, gzab009 (2021).
Campbell, E. C. et al. Laboratory evolution of protein conformational dynamics. Curr. Opin. Struct. Biol. 50, 49–57 (2018).
Maria-Solano, M. A., Serrano-Hervás, E., Romero-Rivera, A., Iglesias-Fernández, J. & Osuna, S. Role of conformational dynamics in the evolution of novel enzyme function. Chem. Commun. 54, 6622–6634 (2018).
Crean, R. M., Gardner, J. M. & Kamerlin, S. C. L. Harnessing conformational plasticity to generate designer enzymes. J. Am. Chem. Soc. 142, 11324–11342 (2020).
Pinto, G. P., Corbella, M., Demkiv, A. O. & Kamerlin, S. C. L. Exploiting enzyme evolution for computational protein design. Trends Biochem. Sci. 47, 375–389 (2021).
Brown, F. K. & Kollman, P. A. Molecular dynamics simulations of “loop closing” in the enzyme triose phosphate isomerase. J. Mol. Biol. 198, 533–546 (1987).
Joseph, D., Petsko, G. & Karplus, M. Anatomy of a conformational change: hinged “lid” motion of the triosephosphate isomerase loop. Science 249, 1425–1428 (1990).
Williams, J. C. & McDermott, A. E. Dynamics of the flexible loop of triose-phosphate isomerase: the loop motion is not ligand gated. Biochemistry 34, 8309–8319 (1995).
Rozovsky, S., Jogl, G., Tong, L. & McDermott, A. E. Solution-state NMR investigations of triosephosphate isomerase active site loop motion: ligand release in relation to active site loop dynamics. J. Mol. Biol. 310, 271–280 (2001).
Wierenga, R. K., Kapetaniou, E. G. & Venkatesan, R. Triosephosphate isomerase: a highly evolved biocatalyst. Cell. Mol. Life Sci. 67, 3961–3982 (2010).
Papaleo, E. et al. The role of protein loops and linkers in conformational dynamics and allostery. Chem. Rev. 116, 6391–6423 (2016).
Näsvall, J., Sun, L., Roth, J. R. & Andersson, D. I. Real-time evolution of new genes by innovation, amplification and divergence. Science 338, 384–387 (2012).
Newton, W. S. et al. Structural and functional innovations in the real-time evolution of new (βα)8 barrel enzymes. Proc. Natl Acad. Sci. USA 114, 4727–4732 (2017).
Romero-Rivera, A., Corbella, M., Parracino, A., Patrick, W. M. & Kamerlin, S. C. L. Complex loop dynamics underpin activity, specificity and evolvability in the (βα)8 barrel enzymes of histidine and tryptophan biosynthesis. JACS Au 4, 943–960 (2022).
Crean, R. M., Biler, M., van der Kamp, M. W., Hengge, A. C. & Kamerlin, S. C. L. Loop dynamics and enzyme catalysis in protein tyrosine phosphatases. J. Am. Chem. Soc. 143, 3830–3845 (2021).
Moise, G. et al. A YopH PTP1B chimera shows the importance of the WPD-loop sequence to the activity, structure and dynamics of protein tyrosine phosphatases. Biochemistry 57, 5315–5326 (2018).
Whittier, S. K., Hengge, A. C. & Loria, J. P. Conformational motions regulate phosphoryl transfer in related protein tyrosine phosphatases. Science 341, 899–903 (2013).
Shen, R., Crean, R. M., Johnson, S. J., Kamerlin, S. C. L. & Hengge, A. C. Single residue on the WPD-loop affects the pH dependency of catalysis in protein tyrosine phosphatases. JACS Au 5, 646–659 (2021).
Liu, J., Tan, H. & Rost, B. Loopy proteins appear conserved in evolution. J. Mol. Biol. 322, 53–64 (2002).
Gunasekaran, K., Ma, B. & Nussinov, R. Triggering loops and enzyme function: identification of loops that trigger and modulate movements. J. Mol. Biol. 332, 143–159 (2003).
Richard, J. P., Zhai, X. & Malabanan, M. M. Reflections on the catalytic power of a TIM-barrel. Bioorg. Chem. 57, 206–212 (2014).
Richard, J. P. A paradigm for enzyme-catalyzed proton transfer at carbon: triosephosphate isomerase. Biochemistry 51, 2652–2661 (2012).
Richard, J. P. Protein flexibility and stiffness enable efficient enzymatic catalysis. J. Am. Chem. Soc. 141, 3320–3331 (2019).
Richard, J. P., Amyes, T. L. & Reyes, A. C. Orotidine 5′-monophosphate decarboxylase: probing the limits of the possible for enzyme catalysis. Acc. Chem. Res. 51, 960–969 (2018).
He, R., Reyes, A. C., Amyes, T. L. & Richard, J. P. Enzyme architecture: the role of a flexible loop in activation of glycerol-3-phosphate dehydrogenase for catalysis of hydride transfer. Biochemistry 57, 3227–3236 (2018).
Mhashal, A. R. et al. Modeling the role of a flexible loop and active site side chains in hydride transfer catalyzed by glycerol-3-phosphate dehydrogenase. ACS Catal. 19, 11253–11267 (2020).
Ray, W. J., Long, J. W. & Owens, J. D. An analysis of the substrate-induced rate effect in the phosphoglutomuase system. Biochemistry 15, 4006–4017 (1976).
Seelig, B. & Szostak, J. W. Selection and evolution of enzymes from a partially randomized non-catalytic scaffold. Nature 448, 828–831 (2007).
Kaur, G. & Subramanian, S. Repurposing TRASH: emergence of the enzyme organomercurial lyase from a non-catalytic zinc finger scaffold. J. Struct. Biol. 188, 16–21 (2014).
Ortmayer, M. et al. An oxidative N-demethylase reveals PAS transition from ubiquitous sensor to enzyme. Nature 539, 593–597 (2016).
Clifton, B. E. et al. Evolution of cyclohexadienyl dehydratase from an ancestral solute-binding protein. Nat. Chem. Biol. 14, 542–547 (2018).
Risso, V. A. et al. De novo active sites for resurrected Precambrian enzymes. Nat. Commun. 8, 16113 (2017).
Kaltenbach, M. et al. Evolution of chalcone isomerase from a noncatalytic ancestor. Nat. Chem. Biol. 14, 548–555 (2018).
Hedstrom, L., Szilagyi, L. & Rutter, W. J. Converting trypsin to chymotrypsin: the role of surface loops. Science 255, 1249–1253 (1992).
Park, H.-S. et al. Design and evolution of new catalytic activity with an existing protein scaffold. Science 311, 535–538 (2006).
Tawfik, D. S. Loop grafting and the origins of enzyme species. Science 311, 475–576 (2006).
Doucet, N., Watt, E. D. & Loria, J. P. The flexibility of a distant loop modulates active-site motion and product release in ribonuclease A. Biochemistry 48, 7160–7168 (2009).
Clouthier, C. M. et al. Chimeric β-lactamases: global conservation of parental function and fast time-scale dynamics with increased slow motions. PLoS ONE 7, e55283 (2012).
Narayanan, C. et al. Conservation of dynamics associated with biological function in an enzyme superfamily. Structure 26, 426–436 (2018).
Dulcey, C. E., López de los Santos, Y., Létourneau, M., Déziel, E. & Doucet, N. Semi-rational evolution of the 3-(3-hydroxyalkanoyloxy)alkanoate (HAA) synthase RhlA to improve rhamnolipid production in Pseudomonas aeruginosa and Burkholderia glumae. FEBS J. 286, 4036–4059 (2019).
Nestl, B. M. & Hauer, B. Engineering of flexible loops in enzymes. ACS Catal. 4, 3201–3211 (2014).
Shen, R. et al. Insights into the importance of WPD-loop sequence in protein tyrosine phosphatases. Chem. Sci. 13, 13524–13540 (2022).
Hammes-Schiffer, S. & Benkovic, S. J. Relating protein motion to catalysis. Annu. Rev. Biochem. 75, 519–541 (2006).
Tzeng, S.-R. & Kalodimos, C. G. Protein dynamics and allostery: an NMR view. Curr. Opin. Struct. Biol. 21, 62–67 (2011).
Bar-Even, A. et al. The moderately efficient enzyme: evolutionary and physicochemical trends shaping enzyme parameters. Biochemistry 50, 4402–4410 (2011).
Guo, J. & Zhou, H.-X. Protein allostery and conformational dynamics. Chem. Rev. 116, 6503–6515 (2016).
Johansson, K. E. & Lindorff-Larsen, K. Structural heterogeneity and dynamics in protein evolution and design. Curr. Opin. Struct. Biol. 48, 157–163 (2018).
Kreß, N., Halder, J. M., Rapp, L. R. & Hauer, B. Unlocked potential of dynamic elements in protein structures: channels and loops. Curr. Opin. Chem. Biol. 47, 109–116 (2018).
Campitelli, P., Modi, T., Kumar, S. & Banu, O. S. The role of conformational dynamics and allostery in modulating protein evolution. Annu. Rev. Biophys. 49, 267–288 (2020).
Maria-Solano, M. A., Iglesias-Fernández, J. & Osuna, S. Deciphering the allosterically driven conformational ensemble in tryptophan synthase evolution. J. Am. Chem. Soc. 141, 13049–13056 (2019).
Schenkmayerova, A. et al. Engineering the protein dynamics of an ancestral luciferase. Nat. Commun. 12, 3616 (2021).
Qu, G. et al. Unlocking the stereoselectivity and substrate acceptance of enzymes: proline-induced loop engineering test. Angew. Chem. Int. Ed. 61, e202110793 (2021).
Klinman, J. P. & Kohen, A. Evolutionary aspects of enzyme dynamics. J. Biol. Chem. 289, 30205–30212 (2014).
Bhabha, G., Biel, J. T. & Fraser, J. S. Keep on moving: discovering and perturbing the conformational dynamics of enzymes. Acc. Chem. Res. 48, 423–430 (2015).
Pabis, A., Risso, V. A., Sanchez-Ruiz, J. M. & Kamerlin, S. C. L. Cooperativity and flexibility in enzyme evolution. Curr. Opin. Struct. Biol. 48, 83–92 (2018).
Espadaler, J., Querol, E., Aviles, F. X. & Oliva, B. Identification of function-associated loop motifs and appliation to protein function prediction. Bioinformatics 22, 2237–2243 (2006).
Regad, L., Martin, J., Nuel, G. & Camproux, A.-C. Minig protein loops using a structural alphabet and statistical exceptionality. BMC Bioinformat. 11, 75 (2010).
Choi, Y., Agarwal, S. & Deane, C. M. How long is a piece of loop? PeerJ 1, e1 (2013).
Dhar, J. & Chakrabarti, P. Defining the loop structures in proteins based on composite β-turn mimics. Prot. Eng. Des. Sel. 28, 153–161 (2015).
Wierenga, R. K. The TIM-barrel fold: a versatile framework for efficient enzymes. FEBS Lett. 492, 193–198 (2001).
Gerlt, J. A. New wine from old barrels. Nat. Struct. Biol. 7, 171–173 (2000).
Sterner, R. & Höcker, B. Catalytic versatility, stability, and evolution of the (βα)8-barrel enzyme fold. Chem. Rev. 105, 4038–4055 (2005).
Heine, A., Luz, J. G., Wong, C.-H. & Wilson, I. A. Analysis of the class I aldolase binding site architecture based on the crystal structure of 2-deoxyribose-5-phosphate aldolase at 0.99 Å resolution. J. Mol. Biol. 343, 1019–1034 (2004).
Dowling, D. P., Croft, A. K. & Drennan, C. L. Radical use of Rossman and TIM barrel architectures for controling coenzyme B12 chemistry. Annu. Rev. Biophys. 41, 403–427 (2012).
Röthlisberger, D. et al. Kemp elimination catalysts by computational enzyme design. Nature 453, 190–195 (2008).
Jiang, L. et al. De novo computational design of retro-aldol enzymes. Science 319, 1387–1391 (2008).
Claren, J., Malisi, C., Höcker, B. & Sterner, R. Establishing wild-type levels of catalytic activity on natural and artificial (βα)8-barrel protein scaffolds. Proc. Natl Acad. Sci. USA 106, 3704–3709 (2009).
Huang, P.-S. et al. De novo design of a four-fold symmetric TIM-barrel protein with atomic-level accuracy. Nat. Chem. Biol. 12, 29–34 (2016).
Lapidoth, G. et al. Highly active enzymes by automated combinatorial backbone assembly and sequence design. Nat. Commun. 9, 2780 (2018).
Romero-Romero, S., Kordes, S., Michel, F. & Höcker, B. Evolution, folding and design of TIM barrels and related proteins. Curr. Opin. Struct. Biol. 68, 94–104 (2021).
Kadumuri, R. V. & Vadrevu, R. Diversity in αβ and βα loop connections in TIM barrel proteins: implications for stability and design of the fold. Interdiscip. Sci. 10, 805–812 (2018).
Knowles, J. R. Enzyme catalysis: not different, just better. Nature 350, 121–124 (1991).
Knowles, J. R. & Albery, W. J. Perfection in enzyme catalysis: the energetics of triosephosphate isomerase. Acc. Chem. Res. 10, 105–111 (1977).
Albery, W. J. & Knowles, J. R. Evolution of enzyme function and the development of catalytic efficiency. Biochemistry 15, 5631–5640 (1976).
Banner, D. et al. Structure of chicken muscle triose phosphate isomerase determined crystallographically at 2.5 Å resolution: using amino acid sequence data. Nature 255, 609–614 (1975).
Jogl, G., Rozovsky, S., McDermott, A. E. & Tong, L. Optimal alignment for enzymatic proton transfer: structure of the Michaelis complex of triosephosphate isomerase at 1.2-Å resolution. Proc. Natl Acad. Sci. USA 100, 50–55 (2003).
Amyes, T. L. & Richard, J. P. Enzymatic catalysis of proton transfer at carbon: activation of triosephosphate isomerase by phosphite dianion. Biochemistry 46, 5841–5854 (2007).
Go, M. K., Amyes, T. L. & Richard, J. P. Hydron transfer catalyzed by triosephosphate isomerase. Products of the direct and phosphite-activated isomerization of [1-13C]-glycoaldehyde in D2O. Biochemistry 48, 5769–5778 (2009).
Kulkarni, Y. S. et al. The role of ligand-driven conformational changes in enzyme catalysis: modeling the catalytic cage of triosephosphate isomerase. J. Am. Chem. Soc. 140, 3854–3857 (2018).
Malabanan, M. M., Nitsch-Velasquez, L., Amyes, T. L. & Richard, J. P. Magnitude and origin of the enhanced basicity of the catalytic glutamate of triosephosphate isomerase. J. Am. Chem. Soc. 135, 5978–5981 (2013).
Katebi, A. R. & Jernigan, R. L. The critical role of the loops of triosephosphate isomerase for its oligomerization, dynamics and functionality. Prot. Sci. 23, 213–228 (2014).
Liao, Q. et al. Loop motion in triosephosphate isomerase is not a simple open and shut case. J. Am. Chem. Soc. 140, 15889–15903 (2018).
Richard, J. P., Amyes, T. L., Goryanova, B. & Zhai, X. Enzyme architecture: on the importance of being in a protein cage. Curr. Opin. Struct. Biol. 21, 1–10 (2014).
Plach, M. G., Reisinger, B., Sterner, R. & Merkl, R. Long-term persistence of bi-functionality contributes to the robustness of microbial life through exaptation. PLoS Genet. 12, e1005836 (2016).
Due, A. V., Kuper, J., Geerlof, A., von Kries, J. P. & Wilmanns, M. Bisubstrate specificity in histidine/tryptophan biosynthesis isomerase from Mycobacterium tuberculosis by active site metamorphosis. Proc. Natl Acad. Sci. USA 108, 3554–3559 (2011).
Henn-Sax, M. et al. Two (βα)8-barrel enzymes of histidine and tryptophan biosynthesis have similar reaction mechanisms and common strategies for protecting their labile substrates. Biochemistry 41, 12032–12042 (2002).
Söderholm, A. et al. Two-step ligand binding in a (βα)8 barrel enzyme: substrate-bound structures shed new light on the catalytic cycle of HisA. J. Biol. Chem. 290, 24657–24668 (2015).
Hodge, J. E. & Rist, C. E. The Amadori rearrangement under new conditions and its significance for non-enzymatic browning reactions. J. Am. Chem. Soc. 75, 316–322 (1952).
Lundin, E., Näsvall, J. & Andersson, D. I. Mutational pathways and trade-offs between HisA and TrpF functions: implications for evolution via gene duplication and divergence. Front. Microbiol. 11, 588235 (2020).
Lee, C. E., Goodfellow, C., Javid-Majd, F., Baker, E. N. & Lott, J. S. The crystal structure of TrpD, a metabolic enzyme essential for lung colonization by Mycobacterium tuberculosis, in complex with its substrate phosphoribosylpyrophosphate. J. Mol. Biol. 355, 784–797 (2006).
Schlee, S. et al. Kinetic mechanism of indole-3-glycerol phosphate synthase. Biochemistry 52, 132–142 (2013).
Delmer, D. P. & Mills, S. E. Tryptophan synthase from Nicotiana tabacum. Biochim. Biophys. Acta 167, 431–443 (1968).
Miles, E. W., Bauerle, R. & Ahmed, S. A. Tryptophan synthase from Escherichia coli and Salmonella typhimurium. Methods Enzymol. 142, 398–414 (1987).
Dunn, M. F. Allosteric regulation of substrate channeling and catalysis in the tryptophan synthase bienzyme complex. Arch. Biochem. Biophys. 519, 154–166 (2012).
Buller, A. R., van Roye, P., Murciano-Calles, J. & Arnold, F. H. Tryptophan synthase uses an atypical mechanism to achieve substrate specificity. Biochemistry 55, 7043–7046 (2016).
Hyde, C. C., Ahmed, S. A., Padlan, E. A., Miles, E. W. & Davies, D. R. Three-dimensional structure of the tryptophan synthase alpha 2 beta 2 multienzyme complex from Salmonella typhimurium. J. Biol. Chem. 263, 17857–17871 (1998).
Schlee, S. et al. Relationship of catalysis and active site loop dynamics in the (βα)8-barrel enzyme indole-3-glycerol phosphate synthase. Biochemistry 57, 3265–3277 (2018).
Hennig, M., Darimont, B. D., Jansonius, J. N. & Kirchner, K. The catalytic mechanism of indole-3-glycerol phosphate synthase: crystal structures of complexes of the enzyme from Sulfolobus solfataricus with substrate analogue, substrate and product. J. Mol. Biol. 319, 757–766 (2002).
Zaccardi, M. J., Mannweiler, O. & Boehr, D. D. Differences in the catalytic mechanisms of mesophilic and thermophilic indole-3-glycerol phosphate synthase enzymes at their adaptive temperatures. Biochem. Biophys. Res. Commun. 418, 324–329 (2012).
Zaccardi, M. J. et al. Loop–loop interactions govern multiple steps in indole-3-glycerol phosphate synthase catalysis. Prot. Sci. 23, 302–311 (2014).
O’Rourke, K. F., Jelowicki, A. M. & Boehr, D. D. Controlling active site loop dynamics in the (β/α)8 barrel enzyme indole-3-glycerol phosphate synthase. Catalysts 6, 129 (2016).
Schneider, T. R. et al. Loop closure and intersubunit communication in tryptophan synthase. Biochemistry 37, 5394–5406 (1998).
Buller, A. W. et al. Directed evolution of the tryptophan synthase β-subunit for stand-alone function recapitulates allosteric activation. Proc. Natl Acad. Sci. USA 112, 14599–14604 (2015).
Maria-Solano, M. A., Kinateder, T., Iglesias-Fernández, J., Sterner, R. & Osuna, S. In silico identification and experimental validation of distal activity-enhancing mutations in tryptophan synthase. ACS Catal. 11, 13733–13743 (2021).
Romero-Rivera, A., Garcia-Borras, M. & Osuna, S. Role of conformational dynamics in the evolution of retro-aldolase activity. ACS Catal. 7, 8524–8532 (2017).
Gurzov, E. N., Stanley, W. J., Brodnicki, T. C. & Thomas, H. E. Protein tyrosine phosphatases: molecular switches in metabolism and diabetes. Trends Endrocrinol. Metab. 26, 30–39 (2015).
Östman, A., Hellberg, C. & Böhmer, F. D. Protein–tyrosine phosphatases and cancer. Nat. Rev. Cancer 6, 307–320 (2006).
Zhang, Z. Y. Protein–tyrosine phosphatases: biological function, structural characteristics, and mechanism of catalysis. Crit. Rev. Biochem. Mol. Biol. 33, 1–52 (1998).
Brandão, T. A. S., Hengge, A. C. & Johnson, S. J. Insights into the reaction of protein–tyrosine phosphatase 1B. J. Biol. Chem. 285, 15874–15883 (2010).
Wiesmann, C. et al. Allosteric inhibition of protein tyrosine phosphatase 1B. Nat. Struct. Mol. BIol. 11, 730–737 (2004).
Wang, H. et al. A structural exposé of noncanonical molecular reactivity within the protein tyrosine phosphatase WPD-loop. Nat. Commun. 13, 2231 (2022).
Saraste, M., Sibbald, P. R. & Wittinghofer, A. The P-loop — a common motif in ATP- and GTP-binding proteins. Trends Biochem. Sci. 15, 430–434 (1990).
Smith, C. A. & Rayment, I. Active site comparisons highlight structural similarities between myosin and other P-loop proteins. Biophys. J. 70, 1590–1602 (1996).
Tabernero, L., Aricescu, A. R., Jones, E. Y. & Szdlacsek, S. E. Protein tyrosine phosphatases: structure–function relationships. FEBS J. 275, 867–882 (2008).
Pálfy, G., Menyhárd, D. K. & Perczel, A. Dynamically encoded reactivity of Ras enzymes: opening new frontiers for drug discovery. Cancer Metastasis Rev. 39, 1075–1089 (2020).
Pinkston, J. et al. Significant loop motions in the SsoPTP protein tyrosine phosphatase allow for dual general acid functionality. Biochemistry 60, 2888–2901 (2021).
Yun, H.-Y. et al. Structural study reveals the temperature dependent conformational flexibility of Tk-PTP, a protein tyrosine phosphatase from Thermococcus kodakaraensis KOD1. PLoS ONE 13, e0197635 (2018).
Torgeson, K. R. et al. Conserved conformational dynamics determine enzyme activity. Sci. Adv. 8, eabo5546 (2022).
Bush, K. & Jacoby, G. A. Updated functional classification of β-lactamases. Antimicrob. Agents Chemother. 54, 969–976 (2010).
Zou, T., Risso, V. A., Gavira, J. A., Sanchez-Ruiz, J. M. & Ozkan, S. B. Evolution of conformational dynamics determines the conversion of a promiscuous generalist into a specialist enzyme. Mol. Biol. Evol. 32, 132–143 (2015).
Modi, T. et al. Hinge-shift mechanism as a protein design principle for the evolution of β-lactamases from substrate promiscuity to specificity. Nat. Commun. 12, 1852 (2021).
Risso, V. A. et al. Enhancing a de novo enzyme activity by computationally-focused ultra-low-throughput screening. Chem. Sci. 11, 6134–6148 (2020).
Cortina, G. A. & Kasson, P. M. Excess positional mutual information predicts both local and allosteric mutations affecting beta lactamase drug resistance. Bioinformatics 32, 3420–3427 (2016).
Hart, K. M., Ho, C. M. W., Dutta, S., Gross, M. L. & Bowman, G. R. Modeling proteins’ hidden conformations to predict antibiotic resistance. Nat. Commun. 7, 12965 (2016).
Cortina, G. A., Hays, J. M. & Kasson, P. M. Conformational intermediate that controls KPC-2 catalysis and beta-lactam drug resistance. ACS Catal. 8, 2741–2747 (2018).
Knoverek, C. R. et al. Opening of a cryptic pocket in β-lactamase increases penicillinase activity. Proc. Natl Acad. Sci. USA 118, e2106473118 (2021).
Alejaldre, L. et al. Known evolutionary paths are accessible to engineered ß-lactamases having altered protein motions at the timescale of catalytic turnover. Front. Mol. Biosci. 7, 599298 (2020).
Wang, X., Minasov, G. & Shoichet, B. K. Evolution of an antibiotic resistance enzyme constrained by stability and activity trade-offs. J. Mol. Biol. 320, 85–95 (2002).
Salverda, M. L. M., de Visser, J. A. G. M. & Barlow, M. Natural evolution of TEM-1 β-lactamase: experimental reconstruction and clinical relevance. FEMS Microbiol. Rev. 34, 1015–1036 (2010).
Salverda, M. L. M. et al. Initial mutations direct alternative pathways of protein evolution. PLoS Genet. 7, e1001321 (2011).
Fröhlich, C. et al. Cryptic β-lactamase evolution is driven by low β-lactam concentrations. mSphere 6, e00108 (2021).
Galdadas, I. et al. Allosteric communication in class A β-lactamases occurs via cooperative coupling of loop dynamics. eLife 10, e66567 (2021).
Baier, F. & Tokuriki, N. Connectivity between catalytic landscapes of the metallo-β-lactamase superfamily. J. Mol. Biol. 426, 2442–2456 (2014).
Baier, F. et al. Cryptic genetic variation shapes the adaptive evolutionary potential of enzymes. eLife 8, e40789 (2019).
Socha, R. D., Chen, J. & Tokuriki, N. The molecular mechanisms underlying hidden phenotypic variation among metallo-β-lactamases. J. Mol. Biol. 431, 1172–1185 (2019).
Chen, J. Z., Fowler, D. M. & Tokuriki, N. Comprehensive exploration of the translocation, stabiliy and substrate recognition requirements in VIM-2 lactamase. eLife 9, e56707 (2020).
López, C., Delmonti, J., Bonomo, R. A. & Vila, A. J. Deciphering the evolution of metallo-β-lactamases: a journey from the test tube to the bacterial periplasm. J. Biol. Chem. 298, 101665 (2022).
Ortlund, E. A., Birdgham, J. T., Redinbo, M. W. & Thornton, J. W. Crystal structure of an ancient protein: evolution by conformational epistasis. Science 143, 1544–1548 (2007).
Du, J., Say, R. F., Lü, W., Fuchs, G. & Einsle, O. Active-site remodelling in the bifunctional fructose-1,6-bisphosphate aldolase/phosphatase. Nature 478, 534–537 (2011).
Fushinobu, S., Nishimasu, H., Hattori, D., Song, H.-J. & Wakagi, T. Structural basis for the bifunctionality of fructose-1-6,bisphosphate aldolase/phosphatase. Nature 478, 538–541 (2011).
Chesters, C., Wilding, M., Goodall, M. & Micklefield, J. Thermal bifunctionality of bacterial phenylalanine aminomutase and ammonia lyase enzymes. Angew. Chem. Int. Ed. 51, 4344–4348 (2012).
Heinemann, P. M., Armbruster, D. & Hauer, D. Active-site loop variations adjust activity and selectvitiy of the cumene dioxygenase. Nat. Commun. 12, 1095 (2021).
Calvó-Tusell, C., Maria-Solano, M. A., Osuna, S. & Feixas, F. Time evolution of the millisecond allosteric activation of imidazole glycerol phosphate synthase. J. Am. Chem. Soc. 144, 7146–7159 (2022).
Cole, R. & Loria, J. P. Evidence for flexibility in the function of ribonuclease A. Biochemistry 41, 6072–6081 (2002).
Beach, H., Cole, R., Gill, M. & Loria, J. P. Conservation of the mus-ms enzyme motions in the apo- and substrate-mimicked state. J. Am. Chem. Soc. 127, 9167–9176 (2005).
Reich, S., Nestl, B. M. & Hauer, B. Loop-grafted old yellow enzymes in the bienzymatic cascade reduction of allylic alcohols. ChemBioChem 17, 561–565 (2016).
Ripka, J. F., Perez-Riba, A., Chaturbedy, P. K. & Itzhaki, L. S. Testing the length limit of loop grafting in a helical repeat protein. Curr. Res. Struct. Biol. 3, 30–40 (2021).
Planas-Iglesias, J. et al. LoopGrafter: a web tool for transplanting dynamical loops for protein engineering. Nucleic Acids Res. 50, gkac249 (2022).
Afriat-Jurnou, L., Jackson, C. J. & Tawfik, D. S. Reconstructing a missing link in the evolution of a recently diverged phosphotriesterase by active-site loop remodeling. Biochemistry 51, 6047–6055 (2012).
Campbell, E. et al. The role of protein dynamics in the evolution of new enzyme function. Nat. Chem. Biol. 12, 944–950 (2016).
Dodani, S. C. et al. Discovery of a regioselectivity switch in nitrating P450s guided by molecular dynamics simulations and Markov models. Nat. Chem. 8, 419–425 (2016).
Bunzel, H. A. et al. Emergence of a negative activation heat capacity during evolution of a designed enzyme. J. Am. Chem. Soc. 141, 11745–11748 (2019).
Bunzel, H. A. et al. Evolution of dynamical networks enhances catalysis in a designer enzyme. Nat. Chem. 13, 1017–1022 (2021).
Osuna, S. The challenge of predicting distal active site mutations in computational enzyme design. WIREs Comp. Mol. Sci. 11, e1502 (2020).
Boehr, D. D., D’Amico, R. N. & O’Rourke, K. F. Engineered control of enzyme structural dynamics and function. Protein Sci. 27, 825–838 (2018).
Crawford, I. P. Evolution of a biosynthetic pathway: the tryptophan paradigm. Annu. Rev. Microbiol. 43, 567–600 (1989).
Cui, D. S., Lipchock, J. M., Brookner, D. & Loria, J. P. Uncovering the molecular interactions in the catalytic loop that modulate the conformational dynamics in protein tyrosine phosphatase 1B. J. Am. Chem. Soc. 141, 12634–12647 (2019).
Keng, Y. F., Wu, L. & Zhang, Z. Y. Probing the function of the conserved tryptophan in the flexible loop of the Yersinia protein–tyrosine phosphatase. Eur. J. Biochem. 259, 809–814 (1999).
Chaloupkova, R. et al. Light-emitting dehalogenases: reconstruction of multifunctional biocatalysts. ACS Catal. 9, 4810–4823 (2019).
Schenkmayerova, A. et al. Catalytic mechanism for Renilla-type bioluminescence. Nat. Catal. 6, 23–38 (2023).
Loening, A. M., Fenn, T. D. & Gambhir, S. S. Crystal structures of the luciferase and green fluorescent protein from Renilla reniformis. J. Mol. Biol. 374, 1017–1028 (2007).
Oakley, A. J. et al. Crystal structure of haloalkane dehalogenase LinB from Sphingomonas paucimobilis UT26 at 0.95 Å resolution: dynamics of catalytic residues. Biochemistry 43, 870–878 (2004).
Hu, X., Wang, H., Ke, H. & Kuhlman, B. High-resolution design of a protein loop. Proc. Natl Acad. Sci. USA 104, 17668–17673 (2007).
Luscombe, N. M., Austin, S. E., Berman, H. M. & Thornton, J. M. An overview of the structures of protein–DNA complexes. Genome Biol. 1, reviews001 (2000).
Khajehpour, M. et al. Loop dynamics and ligand binding kinetics in the reaction catalyzed by the Yersinia protein tyrosine phosphatase. Biochemistry 46, 4370–4378 (2007).
Peeters, M. C., van Westen, G. J. P., Li, Q. & Ijzerman, A. P. Importance of the extracellular loops in G protein-coupled receptors for ligand recognition and receptor activation. Trends Pharmacol. Sci. 32, 35–42 (2011).
Linse, S., Thulin, E., Nilsson, H. & Stigler, J. Benefits and constraints of covalence: the role of loop length in protein stability and ligand binding. Sci. Rep. 10, 20108 (2020).
Feller, S. M. & Lewitzky, M. What’s in a loop? Cell Commun. Signal. 10, 31 (2012).
Gavenonis, J., Sheneman, B. A., Siegert, T. R., Eshelman, M. R. & Kritzer, J. A. Comprehensive analysis of loops at protein–protein interfaces for macrocycle design. Nat. Chem. Biol. 10, 716–722 (2014).
Siegert, T. R., Bird, M. J., Makwana, K. M. & Kritzer, J. A. Analysis of loops that mediate protein–protein interactions and translation into submicromolar inhibitors. J. Am. Chem. Soc. 138, 12876–12884 (2016).
van Wart, A. T., Durrant, J., Votapka, L. & Amaro, R. E. Weighted implementation of suboptimal paths (WISP): an optimized algorithm and tool for dynamical network analysis. J. Chem. Theory Comput. 10, 511–517 (2014).
Wang, J. et al. Mapping allosteric communications within individual proteins. Nat. Commun. 11, 3862 (2020).
Gerek, Z. N., Kumar, S. & Banu Ozkan, S. Structural dynamics flexibility informs function and evolution at a proteome scale. Evol. Appl. 6, 423–433 (2013).
Radicchi, F., Castellano, C., Cecconi, F., Loreto, V. & Parisi, D. Defining and identifying communities in networks. Proc. Natl Acad. Sci. USA 101, 2658–2663 (2004).
McCammon, J. A. Protein dynamics. Rep. Prog. Phys. 47, 1 (1984).
Bonet, J., Segura, J., Planas-Iglesias, J., Oliva, B. & Fernandez-Fuentes, N. Frag’r’Us: knowledge-based sampling of protein backbone conformations for de novo structure-based protein design. Bioinformatics 30, 1935–1936 (2014).
Nguyen, S. P., Li, Z., Xu, D. & Shang, Y. New deep learning methods for protein loop modeling. IEEE/ACM Trans. Comput. Biol. Bioinformat. 16, 596–606 (2019).
Barozet, A., Molloy, K., Vaisset, M., Siméon, T. & Cortés, J. A reinforcement-learning based approach to enhance exhaustive protein loop sampling. Bioinformatics 36, 1099–1106 (2020).
Pan, F. et al. Protein loop modeling and refinement using deep learning models. Preprint at bioRxiv https://doi.org/10.1101/2021.11.03.467148 (2021).
Jumper, J. et al. Highly accurate protein structure prediction with AlphaFold. Nature 596, 583–589 (2021).
Moffat, L, Greener, J. G. & Jones, D. T. Using AlphaFold for rapid and accurate fixed backbone protein design. Preprint at bioRxiv https://doi.org/10.1101/2021.08.24.457549 (2021).
Acknowledgements
This work was supported by the Knut and Alice Wallenberg Foundation (grant numbers 2018.0140 and 2019.0431). We thank R. Sterner and S. Osuna for providing high-resolution artwork components for Fig. 2.
Author information
Authors and Affiliations
Contributions
All authors researched data for the article. All authors contributed to discussion of the content. All authors wrote, reviewed and edited the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Reviews Chemistry thanks Reinhard Sterner, Bernhard Hauer and Sandra Schlee for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Corbella, M., Pinto, G.P. & Kamerlin, S.C.L. Loop dynamics and the evolution of enzyme activity. Nat Rev Chem 7, 536–547 (2023). https://doi.org/10.1038/s41570-023-00495-w
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41570-023-00495-w
This article is cited by
-
Opportunities and challenges in design and optimization of protein function
Nature Reviews Molecular Cell Biology (2024)
-
Activation and friction in enzymatic loop opening and closing dynamics
Nature Communications (2024)
-
Flexible active-site loops fine-tune substrate specificity of hyperthermophilic metallo-oxidases
JBIC Journal of Biological Inorganic Chemistry (2024)