The Influence of the Partitioning of Sugars, Starch, and Free Proline in Various Organs of Cyclamen graecum on the Biology of the Species and Its Resistance to Abiotic Stressors
Abstract
:1. Introduction
2. Results
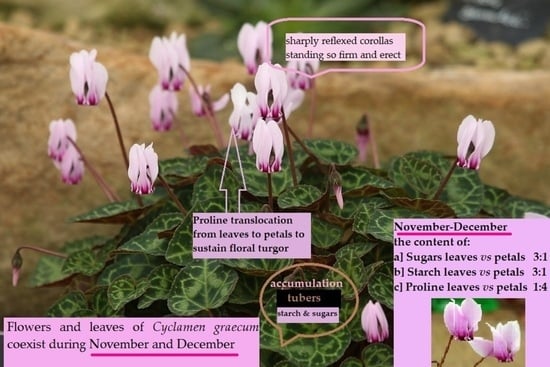
2.1. Phenological Stages
2.2. Sugars
2.3. Starch
2.4. Proline
2.5. Water Status of above-Ground Plant Parts
3. Discussion
4. Material and Methods
4.1. Research Site and Plant Phenology
4.2. Determination of Total Soluble Sugar and Starch
4.3. Determination of Proline
4.4. Determination of Water Status
4.5. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dafni, A.; Cohen, D.; Noy-Mier, I. Life-cycle variation in geophytes. Ann. Mo. Bot. Gard. 1981, 68, 652–660. [Google Scholar] [CrossRef]
- Negbi, M. Theophrastus on geophytes. Bot. J. Linn. Soc. 1989, 100, 15–43. [Google Scholar] [CrossRef]
- Akita, Y.; Ishizaka, H.; Nakayama, M.; Shimada, A.; Kitamura, S.; Hase, Y.; Narumi, I.; Tanaka, A. Comparative analysis of floral pigmentation between wild-type and white-flowered varieties of Cyclamen graecum. J. Hortic. Sci. Biotechnol. 2010, 85, 437–443. [Google Scholar] [CrossRef]
- Grey-Wilson, C. The Genus Cyclamen; Batsford, 2015. Available online: https://www.perlego.com/book/2425483/cyclamen-pdf (accessed on 12 March 2022).
- Mazouz, W.; Djeddi, S. A biological overview on the genus Cyclamen. Eur. J. Sci. Res. 2013, 110, 7–22. Available online: http://www.europeanjournalofscientificresearch.com (accessed on 10 March 2022).
- Panitsa, M.; Trigas, P.; Kontakos, D.; Valli, A.T.; Iatrou, G. Natural and cultural heritage interaction: Aspects of plant diversity in three East Peloponnesian castles (Greece) and conservation evaluation. Plant Biosyst. 2021, 1–9. [Google Scholar] [CrossRef]
- Scarborough, J. Theophrastus on herbals and herbal remedies. J. Hist. Biol. 1978, 11, 353–385. Available online: https://www.jstor.org/stable/4330714 (accessed on 20 February 2022). [CrossRef]
- The Cyclamen Society. Available online: https://www.cyclamen.org/plants/species/ (accessed on 7 April 2022).
- Le Nard, M.; De Hertogh, A.A. Bulb growth and development and flowering. In The Physiology of Flower Bulbs; De Hertogh, A.A., Le Nard, M., Eds.; Elsevier: Amsterdam, The Netherlands, 1993; pp. 29–44. [Google Scholar]
- Dole, J.M. Research approaches for determining cold requirements for forcing and flowering of geophytes. Hort. Sci. 2003, 38, 341–346. [Google Scholar] [CrossRef]
- Adkins, J.A.; Miller, W.B. Storage Organs. In Plant Propagation Concepts and Laboratory Exercises; Beyl, C.A., Trigiano, R.N., Eds.; CRC Press: London, UK, 2008; pp. 303–309. [Google Scholar]
- Clennett, J.C.B. An analysis and revision of Cyclamen L. with emphasis on subgenus Gyrophoebe O. Schwarz. Bot. J. Linn. Soc. 2002, 138, 473–481. [Google Scholar] [CrossRef]
- Liveri, E.; Phitos, D.; Kamari, G. Karyosystematic study of some plant taxa from Greece. Fl. Medit. 2021, 31, 346–354. [Google Scholar] [CrossRef]
- Le Nard, M.; De Hertogh, A.A. General chapter on spring flowering bulbs. In The Physiology of Flower Bulbs; De Hertogh, A.A., Le Nard, M., Eds.; Elsevier: Amsterdam, The Netherlands, 1993; pp. 705–739. [Google Scholar]
- Curuk, P.; Sogut, Z.; Izgu, T.; Sevindik, B.; Tagipur, E.M.; da Silva, J.A.T.; Serce, S.; Solmaz, I.; Kacar, Y.A.; Mendi, N.Y.Y.Y. Morphological characterization of Cyclamen sp. grown naturally in Turkey: Part II. Acta Sci. Pol. Hortorum Cultus 2016, 15, 205–224. [Google Scholar] [CrossRef]
- Chen, L.-Q.; Cheung, L.S.; Feng, L.; Tanner, W.; Frommer, W.B. Transport of sugars. Annu. Rev. Biochem. 2015, 84, 865–894. [Google Scholar] [CrossRef] [PubMed]
- Ruan, Y.-L. Sucrose metabolism: Gateway to diverse carbon use and sugar signaling. Annu. Rev. Plant Biol. 2014, 65, 33–67. [Google Scholar] [CrossRef] [PubMed]
- Szabados, L.; Savouré, A. Proline: A multifunctional amino acid. Trends Plant Sci. 2010, 15, 89–97. [Google Scholar] [CrossRef] [PubMed]
- Harris, S. The Persian Cyclamen. In The Temple of Flora Commentary; The Folio Society: London, UK, 2008; p. 67. [Google Scholar]
- Sherwood, S.; Rix, M. Treasures of Botanical Art; Kew Publishing: Kew, Australia, 2008; pp. 20–21. [Google Scholar]
- Ru, Q.; Wang, X.; Liu, T.; Zheng, H. Physiological and comparative proteomic analyses in response to nitrogen application in an Amaryllidaceae plant, Lycoris aurea. Acta Physiol. Plant. 2013, 35, 271–282. [Google Scholar] [CrossRef]
- Kishor, K.P.B.; Sreenivasulu, N. Is proline accumulation per se correlated with stress tolerance or is proline homeostasis a more critical issue? Plant Cell Environ. 2014, 37, 300–311. [Google Scholar] [CrossRef]
- Mattioli, R.; Costantino, P.; Trovato, M. Proline accumulation in plants-Not only stress. Plant Signal. Behav. 2009, 4, 1016–1018. [Google Scholar] [CrossRef]
- Carter, C.; Shafir, S.; Yehonatan, L.; Palmer, R.G.; Thornburg, R. A novel role for proline in plant floral nectars. Naturwissenschaften 2006, 93, 72–79. [Google Scholar] [CrossRef]
- Kamenetsky, R. Patterns of dormancy and florogenesis in herbaceous perennial plants: Environmental and internal regulation. Crop Sci. 2009, 49, 2400–2404. [Google Scholar] [CrossRef]
- Pouris, J.; Meletiou-Christou, M.S.; Chimona, C.; Rhizopoulou, S. Seasonal functional partitioning of carbohydrates and proline among plant parts of the sand daffodil. Agronomy 2020, 10, 539. [Google Scholar] [CrossRef] [Green Version]
- Orthen, B. Sprouting of the fructan-and starch-storing geophyte Lachenalia minima: Effects on carbohydrate and water content within the bulbs. Physiol. Plant 2001, 113, 308–314. [Google Scholar] [CrossRef]
- Lundgren, M.R.; Des Marais, D.L. Life history variation as a model for understanding trade-offs in plant- environment interactions. Curr. Biol. 2020, 30, R180–R189. [Google Scholar] [CrossRef] [PubMed]
- González, L.; González-Vilar, M. Determination of relative water content. In Handbook of Plant Ecophysiology Techniques; Springer: Dordrecht, The Netherlands, 2011; pp. 207–212. [Google Scholar]
- Rothe, K.; Porzel, A.; Neumann, S.; Grimm, E. Characteristics of the phloem path: Analysis and distribution of carbohydrates in the petiole of Cyclamen. J. Exp. Bot. 1999, 50, 1807–1816. [Google Scholar] [CrossRef]
- Li, W.; Huang, D.; Wang, B.; Hou, X.; Zhang, R.; Yan, M.; Liao, W. Changes of starch and sucrose content and related gene expression during the growth and development of Lanzhou lily bulb. PLoS ONE 2022, 17, e0262506. [Google Scholar] [CrossRef] [PubMed]
- Tarpley, L.; Sassenrath, G.F. Carbohydrate profiles during cotton floral bud (square) development. J. Agron. Crop Sci. 2006, 192, 363–372. [Google Scholar] [CrossRef]
- Beauzamy, L.; Nakayama, N.; Boudaoud, A. Flowers under pressure: Ins and outs of turgor regulation in development. Ann. Bot. 2014, 114, 1517–1533. [Google Scholar] [CrossRef]
- Rhizopoulou, S.; Pantazi, H. Constraints on floral water status of successively blossoming Mediterranean plants under natural conditions. Acta Bot. Gall. 2015, 162, 97–102. [Google Scholar] [CrossRef]
- van Doorn, W.G. Is petal senescence due to sugar starvation? Plant Physiol. 2004, 134, 35–42. [Google Scholar] [CrossRef] [Green Version]
- van Doorn, W.G.; Kamdee, C. Flower opening and closure: An update. J. Exp. Bot. 2014, 65, 5749–5757. [Google Scholar] [CrossRef] [Green Version]
- Khodorova, N.V.; Boitel-Conti, M. The role of temperature in the growth and flowering of geophytes. Plants 2013, 2, 699–711. [Google Scholar] [CrossRef] [Green Version]
- Borghi, M.; Perez de Souza, L.; Yoshida, T.; Fernie, A.R. Flowers and climate change: A metabolic perspective. New Phytol. 2019, 224, 1425–1441. [Google Scholar] [CrossRef] [Green Version]
- Ishizaka, H. Interspecific hybrids of Cyclamen persicum and C. graecum. Euphytica 1996, 91, 109–117. [Google Scholar] [CrossRef]
- Hajihashemi, S.; Brestic, M.; Landi, M.; Skalicky, M. Resistance of Fritillaria imperialis to freezing stress through gene expression, osmotic adjustment and antioxidants. Sci. Rep. 2020, 10, 10427. [Google Scholar] [CrossRef] [PubMed]
- Signorelli, S.; Arellano, J.-B.; Melo, T.-B.; Borsani, O.; Monza, J. Proline does not quench singlet oxygen: Evidence to reconsider its protective role in plants. Plant Physiol. Biochem. 2013, 64, 80–83. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ncube, B.; Finnie, J.F.; Van Staden, J. Carbon–nitrogen ratio and in vitro assimilate partitioning patterns in Cyrtanthus guthrieae L. Plant Physiol. Biochem. 2014, 74, 246–254. [Google Scholar] [CrossRef] [PubMed]
- Tegeder, M.; Masclaux-Daubresse, C. Source and sink mechanisms of nitrogen transport and use. New Phytol. 2018, 217, 35–53. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chiang, H.H.; Dandekar, A.M. Regulation of proline accumulation in Arabidopsis during development and in response to dessication. Plant Cell Environ. 1995, 18, 1280–1290. [Google Scholar] [CrossRef]
- Wainwright, H.; Harwood, A.C. In vitro organogenesis and plant regeneration of Cyclamen persicum Mill. using seedling tissue. J. Hortic. Sci. 1985, 60, 397–403. [Google Scholar] [CrossRef]
- Carfagna, S.; Salbitani, G.; Innangi, M.; Menale, B.; De Castro, O.; Di Martino, C.; Crawford, T.W. Simultaneous biochemical and physiological responses of the roots and leaves of Pancratium maritimum (Amaryllidaceae) to mild salt stress. Plants 2021, 10, 345. [Google Scholar] [CrossRef]
- Larcher, W. Physiological Plant Ecology: Ecophysiology and Stress Physiology of Functional Groups; Springer: Berlin, Germany, 2003; p. 233. [Google Scholar]
- Proietti, S.; Scariot, V.; De Pascale, S.; Paradiso, R. Flowering mechanisms and environmental stimuli for flower transition: Bases for production scheduling in greenhouse floriculture. Plants 2022, 11, 432. [Google Scholar] [CrossRef]
- Ghosh, U.K.; Islam, M.N.; Siddiqui, M.N.; Cao, X.; Khan, M.A.R. Proline, a multifaceted signaling molecule in plant responses to abiotic stress: Understanding the physiological mechanisms. Plant Biol. 2022, 24, 227–239. [Google Scholar] [CrossRef]
- Schwacke, R.; Grallath, S.; Breitkreuz, K.E.; Stransky, E.; Stransky, H.; Frommer, W.B.; Rentsch, D. LeProT1, a transporter for proline, glycine betaine, and gamma-amino butyric acid in tomato pollen. Plant Cell 1999, 11, 377–392. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Funck, D.; Winter, G.; Baumgarten, L.; Forlani, G. Requirement of proline synthesis during Arabidopsis reproductive development. BMC Plant Biol. 2012, 12, 191. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ishizaka, H. Cytogenetic studies in Cyclamen persicum, C. graecum (Primulaceae) and their hybrids. Plant Syst. Evol. 2003, 239, 1–14. [Google Scholar] [CrossRef]
- Stewart, C.R. The effect of wilting on proline metabolism in excised bean leaves in the dark. Plant Physiol. 1973, 51, 508–511. [Google Scholar] [CrossRef] [Green Version]
- Margaris, N.S. Structure and dynamics in a phryganic (East Mediterranean) ecosystem. J. Biogeogr. 1976, 3, 249–259. [Google Scholar] [CrossRef]
- Kampouroglou, E.; Economou-Eliopoulos, M. Assessment of the environmental impact by As and heavy metals in lacustrine travertine limestone and soil in Attica, Greece: Mapping of potentially contaminated sites. Catena 2016, 139, 137–166. [Google Scholar] [CrossRef]
- Khalafalla, M.M.; Menesy, F.; Magouz, M.R.; Hamed, E.B. Growth and flowering of endemic wild Libyan geophyte Cyclamen rohlfsianum Ascher, with a high ornamental value. Appl. Ecol. Environ. Res. 2020, 218, 4583–4594. [Google Scholar] [CrossRef]
- Yesson, C.; Culham, A. A phyloclimatic study of Cyclamen. BMC Evol. Biol. 2006, 6, 72. [Google Scholar] [CrossRef] [Green Version]
- Leven, S. Cyclamen graecum. 2004. Available online: https://www.srgc.org.uk/monthfeature/nov2004/content.html (accessed on 5 March 2022).
- Debussche, M.; Garnier, E.; Thompson, J.D. Exploring the causes of variation in phenology and morphology in Mediterranean geophytes: A genus-wide study of Cyclamen. Bot. J. Linn. Soc. 2004, 145, 469–484. [Google Scholar] [CrossRef] [Green Version]
- Dubois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.A.; Smith, F. Colorimetric method for determination of sugars and related substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Buysse, J.; Merckx, R. An improved colorimetric method to quantify sugar content of plant tissue. J. Exp. Bot. 1993, 4, 1627–1629. [Google Scholar] [CrossRef]
- Meletiou-Christou, M.S.; Rhizopoulou, S. Leaf functional traits of four evergreen species growing in Mediterranean environmental conditions. Acta Physiol. Plant. 2017, 39, 34. [Google Scholar] [CrossRef]
- Bates, L.S.; Waldren, R.P.; Teare, I.D. Rapid determination of free proline for water studies. Plant Soil 1973, 39, 205–207. [Google Scholar] [CrossRef]
- Ain-Lhout, F.; Zunzunegui, M.; Barradas, M.D.; Tirado, R.; Clavijo, A.; Novo, F.G. Comparison of proline accumulation in two Mediterranean shrubs subjected to natural and experimental water deficit. Plant Soil 2001, 230, 175–183. [Google Scholar] [CrossRef]
- Richter, H. A diagram for the description of water relations in plant cells and organs. J. Exp. Bot. 1978, 29, 1197–1203. [Google Scholar] [CrossRef]
- Rhizopoulou, S.; Meletiou-Christou, M.S.; Diamantoglou, S. Water relations for sun and shade leaves of four Mediterranean evergreen sclerophylls. J. Exp. Bot. 1991, 42, 627–635. [Google Scholar] [CrossRef]

 ), leaves (
), leaves (  ) and tubers (
) and tubers (  )), i.e., the active phase indicated by the ivory area that includes flower initiation and longevity (September–December), and leaf emergence, development, longevity, and senescence (November–April), and the dormant phase indicated by the gray area that includes only the subterranean tubers, because above-ground growth is not visible.
)), i.e., the active phase indicated by the ivory area that includes flower initiation and longevity (September–December), and leaf emergence, development, longevity, and senescence (November–April), and the dormant phase indicated by the gray area that includes only the subterranean tubers, because above-ground growth is not visible.
 ), leaves (
), leaves (  ) and tubers (
) and tubers (  )), i.e., the active phase indicated by the ivory area that includes flower initiation and longevity (September–December), and leaf emergence, development, longevity, and senescence (November–April), and the dormant phase indicated by the gray area that includes only the subterranean tubers, because above-ground growth is not visible.
)), i.e., the active phase indicated by the ivory area that includes flower initiation and longevity (September–December), and leaf emergence, development, longevity, and senescence (November–April), and the dormant phase indicated by the gray area that includes only the subterranean tubers, because above-ground growth is not visible.




| Months | Ψ (MPa) | Ψs (MPa) | Ψp (MPa) |
|---|---|---|---|
| September | −1.08 ± 0.02 d | −1.18 ± 0.05 d | 0.10 ± 0.03 b,c |
| October | −0.74 ± 0.04 c | −0.97 ± 0.02 c | 0.23 ± 0.03 a |
| November | −0.56 ± 0.05 b | −0.72 ± 0.04 b | 0.16 ± 0.04 b |
| December | −0.40 ± 0.02 a | −0.46 ± 0.03 a | 0.06 ± 0.02 c |
| Months | RWC |
|---|---|
| November | 79.34 ± 0.06 a |
| December | 78.83 ± 0.10 a |
| January | 77.33 ± 0.14 a |
| February | 77.15 ± 0.08 a |
| March | 77.23 ± 0.05 a |
| April | 68.38 ± 0.12 b |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pouris, J.; Levizou, E.; Karatassiou, M.; Meletiou-Christou, M.-S.; Rhizopoulou, S. The Influence of the Partitioning of Sugars, Starch, and Free Proline in Various Organs of Cyclamen graecum on the Biology of the Species and Its Resistance to Abiotic Stressors. Plants 2022, 11, 1254. https://doi.org/10.3390/plants11091254
Pouris J, Levizou E, Karatassiou M, Meletiou-Christou M-S, Rhizopoulou S. The Influence of the Partitioning of Sugars, Starch, and Free Proline in Various Organs of Cyclamen graecum on the Biology of the Species and Its Resistance to Abiotic Stressors. Plants. 2022; 11(9):1254. https://doi.org/10.3390/plants11091254
Chicago/Turabian StylePouris, John, Efi Levizou, Maria Karatassiou, Maria-Sonia Meletiou-Christou, and Sophia Rhizopoulou. 2022. "The Influence of the Partitioning of Sugars, Starch, and Free Proline in Various Organs of Cyclamen graecum on the Biology of the Species and Its Resistance to Abiotic Stressors" Plants 11, no. 9: 1254. https://doi.org/10.3390/plants11091254