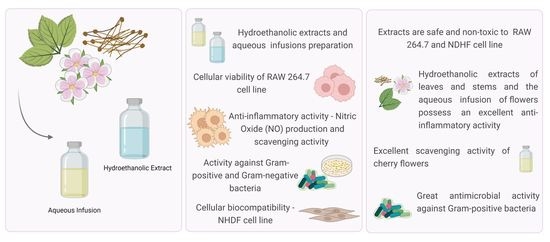
Anti-Inflammatory and Antimicrobial Activities of Portuguese Prunus avium L. (Sweet Cherry) By-Products Extracts
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Plant Material
2.3. Extracts Preparation
2.4. Cell Culture
2.5. Anti-Inflammatory Potential
2.5.1. Effect of Cherry By-Products Extracts on Cellular Viability
2.5.2. Nitric Oxide (NO) Radicals Production
2.5.3. Determination of Radicals NO Scavenging Activity
2.6. Antimicrobial Potential
2.6.1. Bacterial Strains and Growth Conditions
2.6.2. Disk Diffusion Method
2.6.3. Evaluation of the Minimum Inhibitory Concentration (MIC)
2.7. Evaluation of P. avium by-Products Biocompatibility
2.8. Statistical Analysis
3. Results and Discussion
3.1. Anti-Inflammatory Potential
3.1.1. Effect of P. avium By-Products on the Viability of the Raw 264.7 Cell Line
3.1.2. Effect of P. avium By-Products on NO Production
3.1.3. Effect of P. avium By-Products on NO Scavenging Activity
3.2. Antimicrobial Potential
3.3. Effect of P. avium By-Products on NHDF Cell Line
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Poswal, F.S.; Russell, G.; Mackonochie, M.; MacLennan, E.; Adukwu, E.C.; Rolfe, V. Herbal Teas and their Health Benefits: A Scoping Review. Plant Foods Hum. Nutr. 2019, 74, 266–276. [Google Scholar] [CrossRef]
- Ahnen, R.T.; Jonnalagadda, S.S.; Slavin, J.L. Role of plant protein in nutrition, wellness, and health. Nutr. Rev. 2019, 77, 735–747. [Google Scholar] [CrossRef]
- Barreira, J.C.M.; Ferreira, I.C.F.R.; Oliveira, M.B.P.P.; Pereira, J.A. Effects of different phenols extraction conditions on antioxidant activity of almond (prunus dulcis) fruits. J. Food Biochem. 2009, 33, 763–776. [Google Scholar] [CrossRef]
- Ferreira-Santos, P.; Zanuso, E.; Genisheva, Z.; Rocha, C.M.R.; Teixeira, J.A. Green and Sustainable Valorization of Bioactive Phenolic Compounds from Pinus By-Products. Molecules 2020, 25, 2931. [Google Scholar] [CrossRef]
- Chockchaisawasdee, S.; Golding, J.B.; Vuong, Q.V.; Papoutsis, K.; Stathopoulos, C.E. Sweet cherry: Composition, postharvest preservation, processing and trends for its future use. Trends Food Sci. Technol. 2016, 55, 72–83. [Google Scholar] [CrossRef]
- Gonçalves, A.C.; Bento, C.; Jesus, F.; Alves, G.; Silva, L.R. Sweet cherry phenolic compounds: Identification, characterization, and health benefits. In Studies in Natural Products Chemistry; Atta-ur-Rahman, F., Ed.; Science Publishers: Amesterdam, The Netherlands, 2018; pp. 31–78. ISBN 9780444641793. [Google Scholar]
- Nunes, A.R.; Gonçalves, A.C.; Falcão, A.; Alves, G.; Silva, L.R. Prunus avium L. (Sweet Cherry) By-Products: A Source of Phenolic Compounds with Antioxidant and Anti-Hyperglycemic Properties—A Review. Appl. Sci. 2021, 11, 8516. [Google Scholar] [CrossRef]
- Gonçalves, A.C.; Rodrigues, M.; Flores-Félix, J.D.; Nunes, A.R.; Ribeiro, A.B.; Alves, G. Sweet cherry phenolics revealed to be promising agents in inhibiting P-glycoprotein activity and increasing cellular viability under oxidative stress conditions: In vitro and in silico study. J. Food Sci. 2021, 87, 450–465. [Google Scholar] [CrossRef]
- Gonçalves, A.C.; Campos, G.; Alves, G.; García-Viguera, C.; Moreno, D.A.; Silva, L.R. Physical and phytochemical compositon of 23 Portuguese sweet cherries as conditioned by variety (or genotype). Food Chem. 2021, 335, 127637. [Google Scholar] [CrossRef]
- Afonso, S.; Oliveira, I.V.; Meyer, A.S.; Aires, A.; Saavedra, M.J.; Gonçalves, B. Phenolic Profile and Bioactive Potential of Stems and Seed Kernels of Sweet Cherry Fruit. Antioxidants 2020, 9, 1295. [Google Scholar] [CrossRef]
- Gonçalves, A.C.; Costa, A.R.; Flores-Félix, J.D.; Falcão, A.; Alves, G.; Silva, L.R. Anti-Inflammatory and Antiproliferative Properties of Sweet Cherry Phenolic-Rich Extracts. Molecules 2022, 27, 268. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, A.C.; Campos, G.; Pinto, E.; Oliveira, A.S.; Almeida, A.; de Pinho, P.G.; Alves, G.; Silva, L.R. Essential and non-essential elements, and volatile organic compounds for the discrimination of twenty-three sweet cherry cultivars from Fundão, Portugal. Food Chem. 2022, 367, 130503. [Google Scholar] [CrossRef] [PubMed]
- Nunes, A.R.; Gonçalves, A.C.; Alves, G.; Falcão, A.; García-Viguera, C.; Moreno, D.A.; Silva, L.R. Valorisation of Prunus avium L. By-Products: Phenolic Composition and Effect on Caco-2 Cells Viability. Foods 2021, 10, 1185. [Google Scholar] [CrossRef]
- Nunes, A.R.; Gonçalves, A.C.; Pinto, E.; Amaro, F.; Flores-Félix, J.D.; Almeida, A.; de Pinho, P.G.; Falcão, A.; Alves, G.; Silva, L.R. Mineral Content and Volatile Profiling of Prunus avium L. (Sweet Cherry) By-Products from Fundão Region (Portugal). Foods 2022, 11, 751. [Google Scholar] [CrossRef]
- Bastos, C.; Barros, L.; Duenas, M.; Calhelha, R.C.; Queiroz, M.J.R.P.; Santos-Buelga, C.; Ferreira, I.C.F.R. Chemical characterisation and bioactive properties of Prunus avium L.: The widely studied fruits and the unexplored stems. Food Chem. 2015, 173, 1045–1053. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hussain, T.; Tan, B.; Yin, Y.; Blachier, F.; Tossou, M.C.B.; Rahu, N. Oxidative Stress and Inflammation: What Polyphenols Can Do for Us? Oxid. Med. Cell. Longev. 2016, 2016, 7432797. [Google Scholar] [CrossRef] [Green Version]
- Shahidi, F.; Yeo, J. Bioactivities of Phenolics by Focusing on Suppression of Chronic Diseases: A Review. Int. J. Mol. Sci. 2018, 19, 1573. [Google Scholar] [CrossRef] [Green Version]
- Abedini, A.; Colin, M.; Hubert, J.; Charpentier, E.; Angelis, A.; Bounasri, H.; Bertaux, B.; Kotland, A.; Reffuveille, F.; Nuzillard, J.-M.; et al. Abundant Extractable Metabolites from Temperate Tree Barks: The Specific Antimicrobial Activity of Prunus Avium Extracts. Antibiotics 2020, 9, 111. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gonçalves, A.C.; Rodrigues, M.; Santos, A.; Alves, G.; Silva, L.R. Antioxidant Status, Antidiabetic Properties and Effects on Caco-2 Cells of Colored and Non-Colored Enriched Extracts of Sweet Cherry Fruits. Nutrients 2018, 10, 1688. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oliveira, A.S.; Rolo, J.; Gaspar, C.; Cavaleiro, C.; Salgueiro, L.; Palmeira-de-Oliveira, R.; Ferraz, C.; Coelho, S.; Pastorinho, M.R.; Sousa, A.C.; et al. Chemical characterization and bioactive potential of Thymus×citriodorus (Pers.) Schreb. preparations for anti-acne applications: Antimicrobial, anti-biofilm, anti-inflammatory and safety profiles. J. Ethnopharmacol. 2022, 287, 114935. [Google Scholar] [CrossRef]
- Jesus, F.; Gonçalves, A.C.; Alves, G.; Silva, L.R. Exploring the phenolic profile, antioxidant, antidiabetic and anti-hemolytic potential of Prunus avium vegetal parts. Food Res. Int. 2019, 116, 600–610. [Google Scholar] [CrossRef]
- Martins, J.M.; Scheffer, M.C.; de Melo Machado, H.; Schörner, M.A.; Golfetto, L.; dos Santos, T.M.; Barazzetti, F.H.; de Albuquerque, V.C.B.; Bazzo, M.L. Spectinomycin, gentamicin, and routine disc diffusion testing: An alternative for the treatment and monitoring of multidrug-resistant Neisseria gonorrhoeae? J. Microbiol. Methods 2022, 197, 106480. [Google Scholar] [CrossRef] [PubMed]
- Coimbra, A.; Miguel, S.; Ribeiro, M.; Coutinho, P.; Silva, L.; Duarte, A.P.; Ferreira, S. Thymus zygis Essential Oil: Phytochemical Characterization, Bioactivity Evaluation and Synergistic Effect with Antibiotics against Staphylococcus aureus. Antibiotics 2022, 11, 146. [Google Scholar] [CrossRef] [PubMed]
- Coimbra, A.T.; Luís, Â.; Batista, M.T.; Ferreira, S.; Duarte, A.P. Phytochemical Characterization, Bioactivities Evaluation and Synergistic Effect of Arbutus unedo and Crataegus monogyna Extracts with Amphotericin B. Curr. Microbiol. 2020, 77, 2143–2154. [Google Scholar] [CrossRef]
- Chen, L.; Deng, H.; Cui, H.; Fang, J.; Zuo, Z.; Deng, J.; Li, Y.; Wang, X.; Zhao, L. Inflammatory responses and inflammation-associated diseases in organs. Oncotarget 2018, 9, 7204–7218. [Google Scholar] [CrossRef] [Green Version]
- Orabona, C.; Orecchini, E.; Volpi, C.; Bacaloni, F.; Panfili, E.; Pagano, C.; Perioli, L.; Belladonna, M.L. Crocus sativus L. Petal Extract Inhibits Inflammation and Osteoclastogenesis in RAW 264.7 Cell Model. Pharmaceutics 2022, 14, 1290. [Google Scholar] [CrossRef] [PubMed]
- Zaynab, M.; Fatima, M.; Abbas, S.; Sharif, Y.; Umair, M.; Zafar, M.H.; Bahadar, K. Role of secondary metabolites in plant defense against pathogens. Microb. Pathog. 2018, 124, 198–202. [Google Scholar] [CrossRef]
- Ferretti, G.; Bacchetti, T.; Belleggia, A.; Neri, D. Cherry antioxidants: From farm to table. Molecules 2010, 15, 6993–7005. [Google Scholar] [CrossRef]
- Maleki, S.J.; Crespo, J.F.; Cabanillas, B. Anti-inflammatory effects of flavonoids. Food Chem. 2019, 299, 125124. [Google Scholar] [CrossRef]
- Chang, S.K.; Alasalvar, C.; Shahidi, F. Superfruits: Phytochemicals, antioxidant efficacies, and health effects—A comprehensive review. Crit. Rev. Food Sci. Nutr. 2019, 59, 1580–1604. [Google Scholar] [CrossRef]
- Afonso, A.F.; Pereira, O.R.; Fernandes, Â.S.F.; Calhelha, R.C.; Silva, A.M.S.; Ferreira, I.C.F.; Cardoso, S.M. The Health-Benefits and Phytochemical Profile of Salvia apiana and Salvia farinacea var. Victoria Blue Decoctions. Antioxidants 2019, 8, 241. [Google Scholar] [CrossRef]
- Xu, X.; Yin, P.; Wan, C.; Chong, X.; Liu, M.; Cheng, P.; Chen, J.; Liu, F.; Xu, J. Punicalagin inhibits inflammation in LPS-induced RAW264.7 macrophages via the suppression of TLR4-mediated MAPKs and NF-κB activation. Inflammation 2014, 37, 956–965. [Google Scholar] [CrossRef] [PubMed]
- Al-Khayri, J.M.; Sahana, G.R.; Nagella, P.; Joseph, B.V.; Alessa, F.M.; Al-Mssallem, M.Q. Flavonoids as Potential Anti-Inflammatory Molecules: A Review. Molecules 2022, 27, 2901. [Google Scholar] [CrossRef] [PubMed]
- Seeram, N.P.; Momin, R.A.; Nair, M.G.; Bourquin, L.D. Cyclooxygenase inhibitory and antioxidant cyanidin glycosides in cherries and berries. Phytomedicine 2001, 8, 362–369. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.O.; Min, K.J.; Kwon, T.K.; Um, B.H.; Moreau, R.A.; Choi, S.W. Anti-inflammatory activity of hydroxycinnamic acid derivatives isolated from corn bran in lipopolysaccharide-stimulated Raw 264.7 macrophages. Food Chem. Toxicol. 2012, 50, 1309–1316. [Google Scholar] [CrossRef] [PubMed]
- Jung, H.; Kwak, H.-K.; Hwang, K.T. Antioxidant and antiinflammatory activities of cyanidin-3-glucoside and cyanidin-3-rutinoside in hydrogen peroxide and lipopolysaccharide-treated RAW264.7 cells. Food Sci. Biotechnol. 2014, 23, 2053–2062. [Google Scholar] [CrossRef]
- Tang, J.; Diao, P.; Shu, X.; Li, L.; Xiong, L. Quercetin and Quercitrin Attenuates the Inflammatory Response and Oxidative Stress in LPS-Induced RAW264.7 Cells: In Vitro Assessment and a Theoretical Model. Biomed Res. Int. 2019, 2019, 7039802. [Google Scholar] [CrossRef] [Green Version]
- Smailagić, A.; Ristivojević, P.; Dimkić, I.; Pavlović, T.; Dabić Zagorac, D.; Veljović, S.; Fotirić Akšić, M.; Meland, M.; Natić, M. Radical Scavenging and Antimicrobial Properties of Polyphenol Rich Waste Wood Extracts. Foods 2020, 9, 319. [Google Scholar] [CrossRef] [Green Version]
- Aires, A.; Dias, C.; Carvalho, R.; Saavedra, M.J. Analysis of glycosylated flavonoids extracted from sweet-cherry stems, as antibacterial agents against pathogenic Escherichia coli isolates. Acta Biochim. Pol. 2017, 64, 265–271. [Google Scholar] [CrossRef]
- Liu, J.; Du, C.; Beaman, H.T.; Monroe, M.B.B. Characterization of Phenolic Acid Antimicrobial and Antioxidant Structure-Property Relationships. Pharmaceutics 2020, 12, 419. [Google Scholar] [CrossRef]
- Lou, Z.; Wang, H.; Zhu, S.; Ma, C.; Wang, Z. Antibacterial activity and mechanism of action of chlorogenic acid. J. Food Sci. 2011, 76, M398–M403. [Google Scholar] [CrossRef]
- Nunes, R.; Pasko, P.; Tyszka-Czochara, M.; Szewczyk, A.; Szlosarczyk, M.; Carvalho, I.S. Antibacterial, antioxidant and anti-proliferative properties and zinc content of five south Portugal herbs. Pharm. Biol. 2017, 55, 114–123. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gullon, B.; Pintado, M.E.; Pérez-Álvarez, J.A.; Viuda-Martos, M. Assessment of polyphenolic profile and antibacterial activity of pomegranate peel (Punica granatum) flour obtained from co-product of juice extraction. Food Control 2016, 59, 94–98. [Google Scholar] [CrossRef]
- Cuelho, C.H.F.; Alves, G.A.D.; Lovatto, M.O.; Bonilha, I.F.; Barbisan, F.; da Cruz, I.B.M.; Oliveira, S.M.; Fachinetto, R.; do Canto, G.S.; Manfron, M.P. Topical formulation containing Ilex Paraguariensis extract increases metalloproteinases and myeloperoxidase activities in mice exposed to UVB radiation. J. Photochem. Photobiol. B. 2018, 189, 95–103. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Lee, C.-W.; Kim, E.K.; Lee, S.-J.; Park, N.-H.; Kim, H.-S.; Kim, H.-K.; Char, K.; Jang, Y.P.; Kim, J.-W. Inhibition effect of Gynura procumbens extract on UV-B-induced matrix-metalloproteinase expression in human dermal fibroblasts. J. Ethnopharmacol. 2011, 137, 427–433. [Google Scholar] [CrossRef]
- Kurt-Celep, İ.; Celep, E.; Akyüz, S.; İnan, Y.; Barak, T.H.; Akaydın, G.; Telci, D.; Yesilada, E. Hypericum olympicum L. recovers DNA damage and prevents MMP-9 activation induced by UVB in human dermal fibroblasts. J. Ethnopharmacol. 2020, 246, 112202. [Google Scholar] [CrossRef]
- Wu, P.-Y.; Huang, C.-C.; Chu, Y.; Huang, Y.-H.; Lin, P.; Liu, Y.-H.; Wen, K.-C.; Lin, C.-Y.; Hsu, M.-C.; Chiang, H.-M. Alleviation of Ultraviolet B-Induced Photodamage by Coffea arabica Extract in Human Skin Fibroblasts and Hairless Mouse Skin. Int. J. Mol. Sci. 2017, 18, 782. [Google Scholar] [CrossRef] [Green Version]
- Faustino, M.; Veiga, M.; Sousa, P.; Costa, E.M.; Silva, S.; Pintado, M. Agro-Food Byproducts as a New Source of Natural Food Additives. Molecules 2019, 24, 1056. [Google Scholar] [CrossRef]




| Hydroethanolic Extracts | Aqueous Infusions | Control | |||||
|---|---|---|---|---|---|---|---|
| Leaves | Stems | Flowers | Leaves | Stems | Flowers | Gentamycin | |
| Gram-positive | |||||||
| M. luteus CECT 243 | 0.00 ± 0.00 | 1.86 ± 0.25 | 1.00 ± 0.08 | 0.96 ± 0.05 | 1.16 ± 0.19 | 0.00 ± 0.00 | 15.83 ± 0.17 |
| E. faecalis ATCC 29212 | 0.00 ± 0.00 | 2.85 ± 0.23 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.80 ± 0.21 | 0.00 ± 0.00 | 11.05 ± 0.38 |
| B. cereus ATCC 11778 | 1.37 ± 0.17 | 7.24 ± 0.14 | 1.33 ± 0.17 | 1.14 ± 0.03 | 4.06 ± 0.2 | 1.20 ± 0.05 | 15.60 ± 0.82 |
| L. monocytogenes LMG 16779 | 0.00 ± 0.00 | 1.94 ± 0.19 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.99 ± 0.01 | 0.00 ± 0.00 | 15.93 ± 0.13 |
| S. aureus ATCC 25923 | 0.00 ± 0.00 | 3.04 ± 0.16 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.86 ± 0.09 | 0.00 ± 0.00 | 12.93 ± 0.43 |
| B. subtilis CECT 35 | 0.00 ± 0.00 | 0.95 ± 0.06 | 0.76 ± 0.13 | 0.00 ± 0.00 | 0.64 ± 0.16 | 0.00 ± 0.00 | 29.70 ± 0.88 |
| Gram-negative | |||||||
| S. tiphymurium ATCC 13311 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 13.30 ± 0.65 |
| P. aeruginosa ATCC 27853 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 18.03 ± 0.12 |
| E. coli ATCC 25922 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 12.57 ± 0.22 |
| K. pneumoniae ATCC 13883 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 10.30 ± 0.08 |
| P. mirabilis CECT 17 | 0.32 ± 0.15 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 17.73 ± 0.18 |
| S. marcescens CECT 159 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 18.83 ± 0.09 |
| A. baumannii LMG 1025 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 19.33 ± 0.27 |
| Hydroethanolic Extracts | Aqueous Infusions | Negative Control | Positive Control | |||||
|---|---|---|---|---|---|---|---|---|
| Leaves | Stems | Flowers | Leaves | Stems | Flowers | DMSO | Gentamicin | |
| Gram-positive | ||||||||
| M. luteus CECT 243 | 0.5 | 2 | 2 | 2 | 1 | >2 | >2 | 0.016 |
| E. faecalis ATCC 29212 | 0.016 | 0.5 | 2 | 0.016 | 1 | >2 | >2 | 0.016 |
| B. cereus ATCC 11778 | 0.016 | 1 | 2 | 0.016 | 1 | 2 | >2 | 0.016 |
| L. monocytogenes LMG 16779 | 0.016 | 1 | 0.031 | 0.5 | 0.5 | >2 | >2 | 0.016 |
| S. aureus ATCC 25923 | 0.5 | 2 | 2 | 2 | 2 | 2 | >2 | 0.016 |
| B. subtilis CECT 35 | 1 | 2 | >2 | 2 | 2 | >2 | >2 | 0.016 |
| Gram-negative | ||||||||
| S. tiphymurium ATCC 13311 | 0.016 | 0.031 | 2 | 0.016 | 0.031 | 1 | >2 | 0.016 |
| P. aeruginosa ATCC 27853 | 1 | 0.5 | 0.5 | 2 | 0.5 | 2 | >2 | 0.016 |
| E. coli ATCC 25922 | 0.5 | 1 | 1 | 2 | 1 | 2 | >2 | 0.016 |
| K. pneumoniae ATCC 13883 | 1 | 1 | 2 | 0.016 | 1 | >2 | >2 | 0.016 |
| P. mirabilis CECT 17 | 0.016 | 2 | >2 | 1 | 1 | >2 | >2 | 0.016 |
| S. marcescens CECT 159 | 0.016 | 1 | 2 | 0.016 | 1 | >2 | >2 | 0.016 |
| A. baumannii LMG 1025 | 0.016 | 1 | >2 | 0.25 | 1 | 2 | >2 | 0.016 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nunes, A.R.; Flores-Félix, J.D.; Gonçalves, A.C.; Falcão, A.; Alves, G.; Silva, L.R. Anti-Inflammatory and Antimicrobial Activities of Portuguese Prunus avium L. (Sweet Cherry) By-Products Extracts. Nutrients 2022, 14, 4576. https://doi.org/10.3390/nu14214576
Nunes AR, Flores-Félix JD, Gonçalves AC, Falcão A, Alves G, Silva LR. Anti-Inflammatory and Antimicrobial Activities of Portuguese Prunus avium L. (Sweet Cherry) By-Products Extracts. Nutrients. 2022; 14(21):4576. https://doi.org/10.3390/nu14214576
Chicago/Turabian StyleNunes, Ana R., José D. Flores-Félix, Ana C. Gonçalves, Amílcar Falcão, Gilberto Alves, and Luís R. Silva. 2022. "Anti-Inflammatory and Antimicrobial Activities of Portuguese Prunus avium L. (Sweet Cherry) By-Products Extracts" Nutrients 14, no. 21: 4576. https://doi.org/10.3390/nu14214576