Authorship
Gucker, Corey; Shaw, Nancy
Publication Date
November 2022
Nomenclature
Sharpleaf penstemon (Penstemon acuminatus Douglas ex Lindl.) belongs to the Scrophulariaceae family and the coerulei section of the Penstemon genus (Meyer et al. 1995).
Family
Scrophulariaceae – Figwort family
Genus
Penstemon
Species
acuminatus
NRCS Plant Code
PEAC (USDA NRCS 2022).
Subtaxa
Two sharpleaf penstemon varieties are recognized: acuminatus and latebracteatus (N.H. Holmgren) (USDA NRCS 2022).
Common Names
Sharpleaf penstemon, sand or sand-dune penstemon or beardtongue, and St. Joseph’s-wand (Hitchcock and Cronquist 2018; LBJWC 2020).
Chromosome Number
Chromosome number is: 2n = 16 (Hitchcock et al. 1959).
Hybridization
Reviewed literature did not describe hybridization.
Distribution
Sharpleaf penstemon occupies a limited range in central Washington, eastern Oregon, southern and east-central Idaho, northwestern Nevada, and northern Utah (Hitchcock et al. 1959; St. John et al. 2009; Hitchcock and Cronquist 2018). It is most common in low-elevation habitats of the Columbia River Basin in Washington and Oregon and the Snake River Plains of southern Idaho (Hitchcock et al. 1959; Holmgren 1979).
Variety acuminatus occupies the Pacific Northwestern portion of the species range (Hitchcock and Cronquist 2018). It grows in the Columbia River Basin in south-central Washington from southern Douglas County to Klickitat, Benton, and Walla Walla Counties and in adjacent Oregon from Sherman to Wallowa Counties (Holmgren 1979). Variety latebracteatus occupies the Snake River Plain of Idaho and the northwestern Great Basin of Nevada and Utah (Holmgren 1979). It grows from Lincoln, Jerome, and Twin Falls Counties westward to Owyhee, Payette, and Washington Counties in Idaho, southwest through Malheur and Harney Counties of southern Oregon and south through Humboldt and northern Mineral Counties in Nevada (Holmgren 1979).
Habitat And Plant Associations
Sharpleaf penstemon is often associated with sagebrush (Artemisia tridentata) (Fig. 1), antelope bitterbrush (Purshia tridentata), shadscale saltbush (Atriplex confertifolia), and greasewood (Sarcobatus vermiculatus) communities occurring in dry, sandy low-elevation habitats (Hitchcock et al. 1959; Parkinson and DeBolt 2005; Hitchcock and Cronquist 2018; LBJWC 2020).

Figure 1. Sharpleaf penstemon growing along the roadside in big sagebrush habitat in southern Idaho. Photo: USDA Forest Service, Rocky Mountain Research Station (USFS RMRS).
Elevation
The elevation range reported for sharpleaf penstemon is 2,150 to 6,000 ft (650–1,830 m) (St. John et al. 2009). Variety latebracteatus occurs from 1,970 to 4,600 ft (600–1,400 m) in elevation (Holmgren 1979).
Soils
Sharpleaf penstemon grows in well-drained, medium- to coarse-textured soils with a pH of 5 to 8 (St. John et al. 2009; Tilley et al. 2013). It is especially common on sandy plains (Fig. 2) and partially stabilized dunes throughout its range (Pavlik 1985; Taylor 1992; LBJWC 2020).

Figure 2. Sharpleaf penstemon growing on a sandy site near Murphy, ID. Photo: USFS RMRS.
Description
Sharpleaf penstemon is a short-lived perennial with several stout, erect to ascending stems from a short, branched caudex and taproot (Fig. 3) (Hitchcock et al. 1959; Holmgren 1979; Cronquist et al. 1984). Plants can reach 2 ft (0.6 m) tall and produce blue-green, glaucous to glutinous herbage (Holmgren 1979; Cronquist et al. 1984; Taylor 1992; LBJWC 2020). Leaves are entire, thick, and leathery. Basal leaves are generally well-developed, tufted, oblanceolate, and up to 6 in (15 cm) long and 0.8 in (2 cm) wide (Holmgren 1979; Cronquist et al. 1984; Hitchcock and Cronquist 2018). Stem leaves are mostly sessile, clasping, and up to 2.7 in (7 cm) long and 1.4 in (3.5 cm) wide. Leaves become progressively shorter and typically broader up the stem (Hitchcock et al. 1959; Holmgren 1979; Cronquist et al. 1984; Hitchcock and Cronquist 2018).

Figure 3. Sharpleaf penstemon growing near Bruneau, ID. Photo: USFS RMRS.
Flowers are clustered in whorls on long terminal cymes (Fig. 4) (Holmgren 1979; Taylor 1992). Flower clusters are subtended by ovate to suborbicular bracts with overlapping heart-shaped bases. Bracts are usually wider than they are long (Holmgren 1979; Cronquist et al. 1984; Hitchcock and Cronquist 2018). Calyces are up to 9 mm long when in flower and elongate as they mature (Hitchcock et al. 1959; Cronquist et al. 1984). Sepals are narrowly lanceolate (Holmgren 1979). Corollas are bright blue to purple, up to 0.8 in (2 cm) long with tubes that are narrow at the base and wide at the throat (Holmgren 1979; Cronquist et al. 1984; Hitchcock and Cronquist 2018). Corolla lobes are subequal, flare broadly, and often have violet or purple lines inside (Holmgren 1979). Stamens reach the edge of the tube or are slightly exserted. Stamens are glabrous but yellow-bearded for about 1 to 1.5 mm of the tips. Anthers are glabrous and black outside. The anther sacs split completely to become opposite and boat shaped (Holmgren 1979). Seeds are borne in capsules measuring 7 to 13 mm long, with conspicuous, slender beaks up to 5 mm long (Fig. 8). Seeds are rough, angled, brick red to brown, and 1.8 to 3.5 mm long (Hitchcock et al. 1959; Holmgren 1979; Cronquist et al. 1984).

Figure 4. Sharpleaf penstemon inflorescence, note the indeterminate flowering. Photo: USFS RMRS.
Although variety latebracteatus generally produces smaller corollas (11-15 mm), anther sacs (0.7–1.0 mm), and calyces (4.5–6.5 [7.5] mm) than variety acuminatus (corollas: [14] 15-20 mm, anther sacs: 0.9–1.3 [1.5] mm, and calyces 5–10 mm), overlap remains. Distribution (LINK) is the better way to distinguish varieties (Holmgren 1979).
Reproduction
Sharpleaf penstemon reproduces from seed. Pollination is needed for seed production (Fig.5). Flowering commonly occurs from April or May to June or July (Eldredge et al. 2013; Tilley et al. 2013; LBJWC 2020). Variety acuminatus flowers earlier than variety latebracteatus (Holmgren 1979).

Figure 5. Sharpleaf penstemon flower being visited by a honey bee (Apis spp.) at Oregon State University, Malheur Experiment Station in Ontario, OR. Photo: USFS RMRS.
Ecology
Very little is reported on the ecology of sharpleaf penstemon. It is a short-lived (Ogle et al. 2012), shade-intolerant (PFAF 2020) perennial, suggesting it capitalizes on disturbed sites.
Wildlife And Livestock Use
Sharpleaf penstemon is likely important to a variety of small mammals, birds, and insects. It is noted as a potentially important food source of Great Basin pocket mice (Perognathus parvus) in big sagebrush/Sandberg bluegrass (Poa sandbergii) habitats on the Arid Lands Ecology Reserve in Benton County, Washington (O’Farrell et al. 1975). In a review of plant foods used by North American wildlife, seeds of penstemon species are important to western rodents, comprising 10 to 25% of the diets of ground squirrels (Sciuridae), wood rats (Neotoma spp.), and golden-mantled ground squirrels (Callospermophilus lateralis). Finches (Haemorhous spp.) also consume the seeds and pronghorn (Antilocapra americana) feed on the foliage and stems (Martin et al. 1951). In the Cascadia region of Washington and adjacent Oregon, Edith’s checkerspot (Euphydryas editha) utilizes penstemon species as a host plant (James and Nunnallee 2011).
Ethnobotany
Literature indicates that Western tribes used sharpleaf penstemon to treat stomach issues. The Blackfeet used liquid from the boiled plant as a laxative that could relieve stomach cramps. A tea from the leaves stopped vomiting (Johnston 1970). The Shoshone took a tea made from penstemon leaves as a laxative (Van Allen Murphey 1990).
Horticulture
Sharpleaf penstemon is a charming, drought-tolerant landscape plant that attracts pollinators (Nold 1999). Once established, it persists by self-seeding. It grows in USDA hardiness zones 6 to 8 where the annual precipitation averages 10 in (25 cm) or more (St. John et al. 2009; Ogle et al. 2012). It grows best in well-drained dry to moist soils and is cold tolerant but does poorly in prolonged shady and wet conditions (Nold 1999; PFAF 2020). Most penstemon species can be grown from seed if exposed to cold stratification that breaks seed dormancy (Parkinson 2003). This can be facilitated by planting seeds outside in the fall or by keeping seeds moist in a refrigerator or freezer for 3 months prior to planting. Gravel mulch is considered better than bark mulch to promote successful self-seeding as bark holds more moisture, which can cause root rot. Only relatively recently have penstemons become commercially available from local garden centers and catalogs. Popularity of penstemons is rising as the stunning flowers and low water needs are becoming recognized (Parkinson 2003).
Revegetation Use
To date (2022), the use of sharpleaf penstemon in wildland revegetation has been limited, even though it has the potential to improve pollinator habitat at sites with medium to coarse-textured soils receiving 10 to 30 in (25–76 mm) of annual precipitation (Ogle et al. 2012; Tilley et al. 2013). Sharpleaf penstemon is very good for use in revegetation roadsides and stabilizing disturbances in sandy areas. This species is adapted to Major Land Resource Areas (MLRAs) 22A, 23, 24, 25, 26, 27, and 28B, where soils are low in salinity and pH levels range from 6 to 8 (Eldredge et al. 2013).
Sharpleaf penstemon is a short-lived, early- to mid-seral species with low seedling vigor and a moderate growth rate. It supports bee and butterfly populations from May to July (Walker and Shaw 2005; Tilley et al. 2013). It, along with other penstemon species, legumes, and buckwheats (Eriogonum spp.), has been recommended in rotation plantings to provide forage and support for local native pollinator populations brought about from the growth of specific agricultural or conservation cover crops (Eldredge et al. 2013).
Developing A Seed Supply
For restoration to be successful, the right seed needs to be planted in the right place at the right time. Coordinated planning and cooperation is required among partners to first select appropriate species and seed sources and then properly collect, grow, certify, clean, store, and distribute seed for restoration (PCA 2015).
Developing a seed supply begins with seed collection from native stands. Collection sites are determined by current or projected revegetation requirements and goals. Production of nursery stock requires less seed than large-scale seeding operations, which may require establishment of agricultural seed production fields. Regardless of the size and complexity of any revegetation effort, seed certification is essential for tracking seed origin from collection through use (UCIA 2015).
Seed Sourcing
Because empirical seed zones are not currently available for sharpleaf penstemon, generalized provisional seed zones developed by Bower et al. (2014) may be used to select and deploy seed sources. These provisional seed zones identify areas of climatic similarity with comparable winter minimum temperature and aridity (annual heat to moisture index). In Figure 6, Omernik Level III Ecoregions (Omernik 1987) overlay the provisional seeds zones to identify climatically similar but ecologically different areas. For site-specific disturbance regimes and restoration objectives, seed collection locations within a seed zone and ecoregion may be further limited by elevation, soil type, or other factors.
The Western Wildland Environmental Threat Assessment Center’s (USDA FS WWETAC 2017) Threat and Resource Mapping (TRM) Seed Zone application provides links to interactive mapping features useful for seed collection and deployment planning. The Climate Smart Restoration Tool (Richardson et al. 2020) can also guide revegetation planning, seed collection, and seed deployment, particularly when addressing climate change considerations.
Occurrence Map
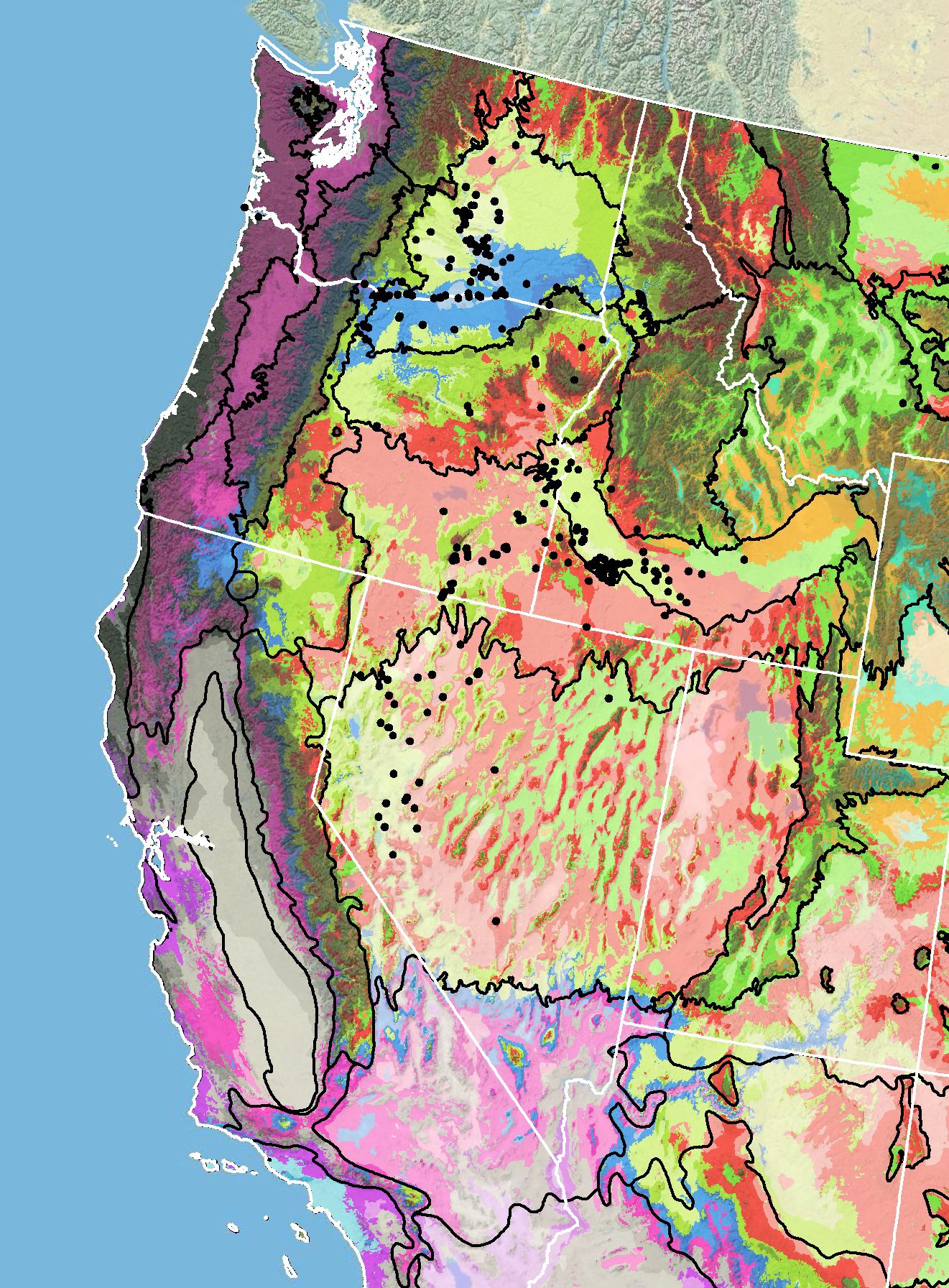
Figure 6. Distribution of sharpleaf penstemon (black circles) based on geo-referenced herbarium specimens and observational data from 1881-2014 (CPNWH 2017; SEINet 2017; USDI USGS 2017). Generalized provisional seed zones (colored regions) (Bower et al. 2014) are overlain by Omernik Level III Ecoregions (black outlines) (Omernik 1987; USDI EPA 2018). Interactive maps, legends, and a mobile app are available (USDA FS WWETAC 2017; www.fs.fed.us/wwetac/threat-map/TRMSeedZoneMapper2.php?). Map prepared by M. Fisk, USDI USGS.
Releases
As of 2022, there were no sharpleaf penstemon germplasm releases.
Wildland Seed Collection
Sharpleaf penstemon phenology varies with elevation, aspect, and seasonal weather (Parkinson and DeBolt 2005), but seed is generally mature when capsules turn tan and begin to open (Fig. 7) (St. John et al. 2009).

Figure 7. Sharpleaf penstemon inflorescences with mature fruits and seeds. Photo: USFS RMRS.
Wildland Seed Certification
Verification of species and tracking of geographic source is necessary whether wildland seed is collected for immediate project use or as stock seed for cultivated increase. This official Source Identification process can be accomplished by following procedures established by the Association of Official Seed Certifying Agencies (AOSCA) Pre-Variety Germplasm Program (Young et al. 2020; UCIA 2015). Wildland seed collectors should become acquainted with state certification agency procedures, regulations, and deadlines in the states where they collect.
If wildland-collected seed is to be sold for direct use in ecological restoration projects, collectors must apply for Source-Identified certification prior to making collections. Pre-collection applications, site inspections, and species and seed amount verification are handled by the AOSCA member state agency where seed collections will be made (see listings at AOSCA.org).
If wildland seed collected by a grower or private collector is to be used as stock seed for planting cultivated seed fields or for nursery propagation (See Agricultural Seed Field Certification section), detailed information regarding collection site and collecting procedures must be provided when applying for certification. Photos and herbarium specimens may be required. Germplasm accessions acquired within established protocols of recognized public agencies, however, are normally eligible to enter the certification process as stock seed without routine certification agency site inspections. For contract grow-outs, however, this collection site information must be provided to the grower to enable certification.
Collection Timing
Wildland seed is mature and ready for harvest when flowering stems and capsules are dry and brown (Figs. 7 and 8). This typically occurs 5 to 8 weeks after flowering (Parkinson and DeBolt 2005; St. John et al. 2009). Seed maturation is quite uniform, and seeds are often released from the capsules within 2 weeks of maturing (Parkinson and DeBolt 2005).
The USDI Bureau of Land Management’s Seeds of Success collection crews made 29 sharpleaf penstemon harvests over 5 years in Idaho (21), Oregon (7), and Nevada (1). Most collections were made in July (20) and the rest in August (9). The earliest harvest date was July 8, 2002, at 3,640 ft (1,110 m) in elevation in Owyhee County, Idaho. The latest harvest date was August 21, 2009, at 3,904 ft (1,190 m) in elevation, in Owyhee County, Idaho. In the single year (2010) with the most collections made (14), the earliest harvest was on July 15 at 2,984 ft (909 m) elevation in Owyhee County, Idaho and the latest was August 13 at 2,800 ft (853 m) in elevation in Owyhee County (USDI BLM SOS 2017).
Collection Methods
Wildland harvests of sharpleaf penstemon are made by clipping mature inflorescences into paper bags or other breathable containers (Parkinson and DeBolt 2005; Barner 2008).
Several collection guidelines and methods should be followed to maximize the genetic diversity of wildland collections: 1) collect seed from a minimum of 50 randomly selected plants; 2) collect from widely separated individuals throughout a population without favoring the most robust or avoiding small stature plants; and 3) collect from all microsites including habitat edges (Basey et al. 2015). General collecting recommendations and guidelines are provided in online manuals (e.g., ENSCONET 2009; USDI BLM SOS 2021). It is critical that wildland seed collection does not impact the sustainability of native plant populations. Collectors should take no more than 20% of the viable seed available at the time of harvest (USDI BLM SOS 2021). Additionally, care must be taken to avoid the inadvertent collection of weedy species, particularly those that produce seeds similar in shape and size to those of sharpleaf penstemon.
Post-Collection Management
Parkinson and DeBolt (2005) recommend thoroughly drying sharpleaf penstemon seed then freezing it for 48 hours to kill insect pests.
Seed Cleaning
Sharpleaf penstemon seed is cleaned by separating seed from the pods then removing fine debris (Meyer et al. 1995; St. John et al. 2009). The USDA Forest Service, Bend Seed Extractory cleaned a small sharpleaf penstemon seed lot (1.1 lbs [0.5 kg]) by first hand-rubbing the stems to remove the seed pods. Pods were then processed through a Westrup Model LA-H laboratory brush machine (Hoffman Manufacturing Inc, Corvallis, OR) using a #7 mantel and medium speed to extract the seed from the pods. Seed was then passed through an air screen cleaner (office Clipper) with medium speed and medium air (Barner 2008). Parkinson and DeBolt (2005) cleaned sharpleaf penstemon seed by first crushing capsules on a rubbing board. Seed and chaff were then screened through sieves with 2 mm and 1 mm square openings (no. 10 and 18 USA STS). Debris was removed using a seed blower (two passes) and then screened again through sieves with 1.7 mm square openings (no. 12 USA STS).

Figure 8. Clean sharpleaf penstemon seeds. Photo: USFS RMRS.
Seed Storage
Sharpleaf penstemon seed is orthodox. Royal Botanic Gardens, Kew reported 90% viability for dry seed stored for 4.2 years at 68 °F (-20 °C) (RBG Kew 2022). The Bend Seed Extractory stored clean dry sharpleaf penstemon seed at 33 to 38 °F (0.5–3 °C) (Barner 2008).
Seed Testing
Procedures for testing the viability and germination of penstemon seeds are provided below.
Viability Testing
The Association of Official Seed Analysts (AOSA) provides a procedure for testing the seed viability of penstemon species (AOSA 2010). Seed is imbibed on moist media overnight at 68 to 77 °F (20–25 °C) then cut longitudinally leaving just enough seed coat at the distal end to keep the halves attached. Cut seeds are soaked in a 0.1 to 0.5% tetrazolium chloride (TZ) for 24 to 48 hrs at 86 to 95 °F (30–35 °C). Seed is considered viable if the entire embryo and endosperm stains evenly. Seed is nonviable when any essential part of the embryo or endosperm is unstained (AOSA 2010).
Purity Testing
Vankus (2006) suggests a minimum of 0.07 oz (2.1 g) of penstemon seed is needed to test purity and noxious weed contamination.
Germination Testing
To test the germination of penstemon species, the AOSA (2016) recommends two methods. Seed is placed on moist blotter paper at 50 to 68 °F (10–20 °C). The first germination count is made at seven days and the last at 14 days for method one. For method two, the first germination count is made at 14 days and the last at 28 days (AOSA 2016).
Germination Biology
Stratification is required for germination of sharpleaf penstemon seed (Meyer et al. 1995; St. John et al. 2009). Chilling duration required was greater for seed collected from a colder, higher elevation site (Twin Falls, ID, 3,700 ft [1,130m]) than the warmer, lower elevation site (Bruneau Sand Dunes, ID, 2,990 ft [910 m]) (Table 1; Meyer et al. 1995). Seed was stored dry (7–9% moisture) at 68 to 72 °F (20–22 °C) until germination testing, which evaluated short- to long-term dark chilling at 34 °F (1 °C) followed by incubation at 50/68 °F (10/20 °C) in 12:12 hr light-dark cycles. For both seed lots, germination was significantly (P < 0.05) improved with stratification. Seed collected from the warmer location germinated at 90% or more after 12 weeks of stratification. Seed collected from the cooler location germinated at 68% after 24 weeks of stratification (Meyer et al. 1995).
Table 1. Percent germination of sharpleaf penstemon seed collected from two sites in Idaho and exposed to increasing chilling duration (Meyer et al. 1995).
| Weeks of chilling (34 °F) | 0 | 4 | 8 | 12 | 16 | 24 |
| Germination (%) | ||||||
| Twin Falls
(Mean Jan temp: 27 °F, Elevation: 3,700 ft) |
0c | 0c | 3bc | 59a | 57a | 68a |
| Bruneau Sand Dunes (Mean Jan temp: 29 °F, Elevation: 2,990 ft) | 0d | 2d | 38bc | 94a | 95a | 97a |
Values within a row followed by different letters are significantly different (P < 0.05).
St. John et al. (2009) suggests seeding sharpleaf penstemon outdoors to stratify seed for 8 to 12 weeks to overcome seed dormancy. The duration of prechilling required for germination has been reduced by watering seeds with liquid smoke or weak gibberellic acid (GA3, 250 ppm) treatments, but seedlings emerging following GA3 treatments were less vigorous than untreated seedlings.
Wildland Seed Yield And Quality
Post-cleaning seed yield and quality of seed lots collected in the Intermountain region are provided in Table 2 (USDA FS BSE 2017). The results indicate that sharpleaf penstemon seed can generally be cleaned to high levels of purity and seed fill and that viability of fresh seed ranges from moderate to high. Seeds/lb reported elsewhere in the literature (400,000–592,380 seeds/lb [881,830–1,305,950 seeds/kg]) fell within the range reported in Table 2 (RMRS calculations from knowns; Walker and Shaw 2005; Vankus 2006; Tilley et al. 2013; RBG Kew 2020).
Table 2. Seed yield and quality of sharpleaf penstemon seed lots collected in the Intermountain region, cleaned by the Bend Seed Extractory, and tested by the Oregon State Seed Laboratory or the USFS National Seed Laboratory (USDA FS BSE 2017).
| Seed lot characteristic | Mean | Range | Samples (no.) |
| Bulk weight (lbs) | 2.56 | 0.04–13.0 | 29 |
| Clean weight (lbs) | 0.41 | 0.02–2.47 | 29 |
| Clean-out ratio | 0.16 | 0.05–0.48 | 29 |
| Purity (%) | 91 | 77–99 | 29 |
| Fill (%)1 | 92 | 80–99 | 29 |
| Viability (%)2 | 92 | 70–98 | 26 |
| Seeds/lb | 487,955 | 378,000–667,000 | 29 |
| Pure live seeds/lb | 411,598 | 290,904–626,884 | 26 |
¹100 seed X-ray test
²Tetrazolium chloride test
Marketing Standards
Acceptable seed purity, viability, and germination specifications vary with revegetation plans. Purity needs are highest for precision seeding equipment used in nurseries, while some rangeland seeding equipment handles less clean seed quite well. Walker and Shaw (2005) suggest seed viability of 84% and purity of 95% for contracted harvests of sharpleaf penstemon seed.
Agricultural Seed Production
Sharpleaf penstemon was grown for seed production at the Oregon State University Malheur Experiment Station (OSU MES). It produced harvestable seed by year 2, and seed yields ranged from 100 to 600 lbs/ac (112–673 kg/ha) in 4 years of evaluation (Shock et al. 2019).
Agricultural Seed Certification
In order to minimize genetic changes in specific accessions of native species when increased in cultivated fields, it is essential to track the geographic source and prevent inadvertent hybridization or selection pressure. This is accomplished by following third party seed certification protocols for Pre-Variety Germplasm (PVG) as established by the Association of Official Seed Certification Agencies (AOSCA). AOSCA members in the U.S., Canada, and other countries administer PVG requirements and standards that track the source and generation of planting stock. Field and cleaning facility inspections then monitor stand establishment, proper isolation distances, control of prohibited weeds, seed harvesting, cleaning, sampling, testing, and labeling for commercial sales (Young et al. 2020; UCIA 2015).
Seed growers apply for certification of their production fields prior to planting and plant only certified stock seed of an allowed generation (usually less than four). The systematic and sequential tracking through the certification process requires preplanning, knowing state regulations and deadlines, and is most smoothly navigated by working closely with state certification agency personnel. See the Wildland Seed Certification section for more information on stock seed sourcing.
Site Preparation
A weed-free, packed soil bed is best for seeding.
Weed Management
Research conducted at the NRCS Aberdeen, Idaho, Plant Materials Center (IDPMC) showed that weeds can be controlled by seeding sharpleaf penstemon in weed-barrier fabric. Planting holes should be 3 to 4 in (8–10 cm) in diameter and spaced 9 to 18 in (23–46 cm) apart (St. John et al. 2009). Weeds were treated chemically in seed production plots at OSU MES. Treatments are described in the Establishment and Growth section, but the success of these treatments was not reported.
Seeding
Sharpleaf penstemon should be seeded in the fall to allow for natural seed scarification. Seeding depths of 0 to 0.6 mm are recommended (St. John et al. 2009). At OSU MES, cold-stratified seed was planted on March 3 using a custom small-plot grain drill. Seed was planted 0.25-in (0.6 cm) deep at 20 to 30 seeds/ft (65–100 seeds/m) of row (Shock et al. 2019). Seed emergence was poor and empty lengths of row were seeded by hand the following October.
Establishment And Growth
Seed production plots at OSU MES were fertilized once but often treated for pests and weeds more once each year (Shock et al. 2019). See Table 3 for the types and frequency of cultural treatments.
Table 3. Fertilization, weed, and pest treatments applied to sharpleaf penstemon seed production plots at OSU MES (Shock et al. 2019).
| Year | Timing | Treatment/ac | Target for control |
| 2006 | Oct 21 | 50 lb P and 2 lb Zn | Feeding |
| 2006 | Nov 17 | Pendimethalin 1 lb ai | Weed control |
| 2007 | May 14 and 29 | Azadirachtim 0.0062 lb ai | Lygus bug control |
| 2007 | Nov 9 | Pendimethalin 1 lb ai | Weed control |
| 2008 | Apr 15 | Pendimethalin 1 lb ai | Weed control |
| 2008 | Apr 15 | Bifenthrin 2EC 0.1 lb ai | Lygus bug control |
| 2009 | Mar 18 | Pendimethalin 1 lb ai AND Clethodim 8 oz |
Weed control |
| 2009 | Dec 4 | Pendimethalin 1 lb ai | Weed control |
Irrigation
Seed yield and plant water relations were evaluated for sharpleaf penstemon at OSU MES over 4 years (2006–2009) (Table 5; Shock et al. 2019). Studies tested additions of 0, 4, and 8 in (0, 100, and 200 mm) of subsurface drip irrigation delivered 12 in (30 cm) deep in four bi-weekly increments starting at the time of flower bud formation (Table 4).
Seed production for sharpleaf penstemon was improved with irrigation only in dry years at OSU MES (Shock et al. 2019). In wet years, seed production was low and stand losses were 12 to 37% more in irrigated than non-irrigated plots. Because of wet fall conditions, sharpleaf penstemon seed was artificially stratified and seeded in the spring of 2005. Emergence was uneven and no plants flowered in 2005. Empty row lengths were seeded by hand in late October 2005. In 2006, the area received 6.4 in (165 mm) of precipitation from March through June and the 64-year average for spring precipitation is 3.6 in (91 mm). Irrigation in 2006 resulted in no significant difference in seed yield (Tables 4 and 5). Seed yield was maximized by 4 in (100 mm) of irrigation in 2007 when spring and winter precipitation was below average. Seed yield showed a linear response to irrigation in 2008, when spring and winter precipitation was again below average. In 2009, seed yields were poor in all plots, and plants were experiencing root rot, which was more pronounced in the irrigated plots. By 2010, substantial lengths of row contained only dead plants and plant death increased with increasing irrigation rate. Stand loss was 51%, 64%, and 89% for the 0-, 4-, and 8-in (0, 100, 200 mm) irrigation treatments, respectively. The trial plots were disked out in 2010. Sharpleaf penstemon performed as a short-lived perennial (Shock et al. 2019).
Table 4. Flowering timing as related to timing of irrigation of sharpleaf penstemon seed production plots growing at Oregon State University’s Malheur Experiment Station in Ontario, OR (Shock et al. 2019).
| Year | Flowering | Irrigation | ||||
| Start | Peak | End | Start | End | Harvest | |
| 2006 | 2 May | 10 May | 19 May | 19 May | 30 June | 7 July |
| 2007 | 19 Apr | —- | 25 May | 19 Apr | 24 June | 9 July |
| 2008 | 29 Apr | —- | 5 June | 29 Apr | 11 June | 11 July |
| 2009 | 2 May | —- | 10 June | 8 May | 12 June | 10 July |
* Seeded March 2005, and areas with low establishment were seeded again in October 2005.
Table 5. Seed yield (lbs/ac) for sharpleaf penstemon in response to no additional irrigation and supplemental irrigation of 4 and 8 in at Oregon State University’s Malheur Experiment Station in Ontario, OR (Shock et al. 2019).
| Year | Supplemental irrigation (in/season*) | ||
| 0 | 4 | 8 | |
| ——Seed yield (lbs/ac)—— | |||
| 2006 | 538.4a** | 611.1a | 544.0a |
| 2007 | 19.3a | 50.1b | 19.1a |
| 2008 | 56.2a | 150.7b | 187.1b |
| 2009 | 20.7a | 12.5a | 11.6a |
*Irrigation season was from bud to seed set and water was delivered through a drip irrigation system.
**Yields within a row followed by different letters are significantly different (P < 0.05).
Pollinator Management
Successful pollination is essential for commercial seed production of sharpleaf penstemon (Stevens et al. 1996). Bees and wasps including sweat bees (Halictidae), bumblebees (Bomus spp.), honey bees (Apis spp.), and leafcutter bees (Megachile spp.) pollinate sharpleaf penstemon flowers (St. John et al. 2009). Management to encourage and sustain native bee populations, when present, can benefit production of native plant crops (Cane 2008). Cavity-nesting honey bees (A. mellifera) can be transported to field locations in portable ground nests or hives where encouraging populations of native bees is not possible (Cane et al. 2012).
Pest Management
Although penstemon seed growers have produced crops without apparent insect damage, if insects do appear, crop damage can be “catastrophic” (Hammon 2016). Penstemon weevils or borer larvae (Hesperobarus ovulum) are limited to southwestern Colorado, but the penstemon clearwing borer (Penstemonia spp.) is widespread and attacks multiple penstemon species (St. John et al. 2009; Hammon 2016). Clearwing borer larvae feed in the stems of the root crown and lower above-ground portions of the plant. A pheromone is available to monitor for adult clearwing borers. Other potentially significant pests include Lygus bugs (Lygus spp.) and raceme-boring moths (St. John et al. 2009). Many Penstemon species are short-lived plants. Hammon (2016) hypothesizes that the short life cycle may be attributed to borers and other insects and pathogens.
Sharpleaf penstemon is also susceptible to soil-borne fusarium and rhizoctonia root rot, these pathogens and their damage is most severe in poorly drained loam or clay soils (St. John et al. 2009). Cucumber mosaic virus was isolated from sharpleaf penstemon plants growing in seed production plots at OSU MES. Infected plants had red foliar ringspots and streaks (Sampangi et al. 2009).
Seed Harvesting
Seed maturation is quite uniform, but capsules begin opening and seed is dispersed rapidly once mature (< 2 weeks) (Parkinson and DeBolt 2005; St. John et al. 2009). Seed is mature when capsules turn brown and begin to open, which is generally 5 to 8 weeks after flowering (St. John et al. 2009). Harvests can be done by hand or direct combining when seeds are mature.
Seed Yields And Stand Life
Based on 4 years of research conducted at OSU MES, sharpleaf penstemon seed was harvested in year 2. Annual seed yields ranged from 20 to 600 lbs/ac (22–670 kg/ha) and averaged 150 to 200 lbs/ac (165–220 kg/ha) (Shock et al. 2019).
Nursery Practice
Researchers who grew sharpleaf penstemon in the greenhouse, reported that proper seed stratification and careful watering were important for success (Parkinson and DeBolt 2005; St. John et al. 2009). St. John et al. (2009) indicated that seed can be stratified naturally by seeding outdoors in fall or winter. Stratification duration was reduced with GA3 treatments, but seedling vigor was reduced (St. John et al. 2009). Parkinson and DeBolt (2005) reported high seedling mortality from damping off. Sharpleaf penstemon seed collected in Elmore County, Idaho (2,680 ft [820 m] in elevation), had 89% viability and 96% purity. Seed was soaked in GA3 (250 ppm) for 24 hours then chilled at 39 °F (4 °C) for up to 60 days. Germination began after 3 days of incubation (constant 70 °F [21 °C], 12/12 hr light-dark cycle) on moist blotter paper and reached 70% by day 4. Germination continued for 48 days, but mold formed after 30 days. Seeds exposed to mold were washed with Thiram. At first signs of germination, seeds were placed in styrofoam cone-tainers filled with a 50:50 mix of peat and vermiculite. Cone-tainers were kept in a greenhouse at 81 °F (27 °C), fertilized minimally and infrequently, and watered automatically when soil saturation fell below 80%. Only 19% of germinants survived. Seedling mortality was caused by damping off. The addition of sand or gravel over the potting mix surface was suggested as a potential remedy (Parkinson and DeBolt 2005).
Wildland Seeding And Planting
To date (2022), use of sharpleaf penstemon in wildland revegetation has been limited, although it has the potential to improve pollinator habitat at sites with medium to coarse-textured soils and disturbed sites with sandy or coarse soils receiving 10 to 30 in (250–760 mm) of annual precipitation (Ogle et al. 2012; Tilley et al. 2013). This species is adapted to MLRAs 22A, 23, 24, 25, 26, 27, and 28B, where soils are low in salinity and pH levels range from 6 to 8 (Eldredge et al. 2013).
It is recommended that sharpleaf penstemon be drill seeded in late fall, 0 to 3 mm deep at a full-stand rate of 3 lbs/ac (3.4 kg/ha) (Ogle et al. 2012; Tilley et al. 2013). In wildland revegetation, sharpleaf penstemon would be a minor component of the seed mix and establishing community; post-seeding management should be based on key species in the establishing community. Grazing should be deferred for at least 2 years in newly seeded areas (St. John et al. 2009).
Sharpleaf penstemon has low seedling vigor, a moderate growth rate, an erect growth form (heights of 8–24 in [20–61 cm]), and medium longevity. Its blue flowers attract butterflies and bees from May to July (Ogle et al. 2012; Eldredge et al. 2013; Tilley et al. 2013), which are important characteristics to consider when developing a seed mix or determining the seeding arrangement.
Acknowledgements
Funding for Western Forbs: Biology, Ecology, and Use in Restoration was provided by the USDI BLM Great Basin Native Plant Materials Ecoregional Program through the Great Basin Fire Science Exchange. Great thanks to the chapter reviewers: Erik Feibert, Oregon State University, Malheur Experiment Station, and Matt Fisk, U.S. Geological Survey.
This research was supported in part by the USDA Forest Service, Rocky Mountain Research Station. The findings and conclusions in this publication are those of the authors and should not be construed to represent any official USDA or U.S. Government determination or policy.
Literature Cited
Association of Official Seed Analysts [AOSA]. 2010. AOSA/SCST Tetrazolium testing handbook. Contribution No. 29. Lincoln, NE: Association of Official Seed Analysts.
Association of Official Seed Analysts [AOSA]. 2016. AOSA rules for testing seeds. Vol. 1. Principles and procedures. Washington, DC: Association of Official Seed Analysts.
Barner, J. 2008. Propagation protocol for production of propagules (seeds, cuttings, poles, etc.) Penstemon acuminatus Douglas ex Lindl. seeds. Native Plant Network. U.S. Department of Agriculture, Forest Service, National Center for Reforestation, Nurseries, and Genetic Resources. http://npn.rngr.net/propagation/protocols [Accessed 2020 June 5].
Basey, A.C.; Fant, J.B.; Kramer, A.T. 2015. Producing native plant materials for restoration: 10 rules to collect and maintain genetic diversity. Native Plants Journal. 16(1): 37-53.
Bower, A.D.; St. Clair, J.B.; Erickson, V. 2014. Generalized provisional seed zones for native plants. Ecological Applications. 24(5): 913-919.
Cane, J.H. 2008. Pollinating bees crucial to farming wildflower seed for U.S. habitat restoration. In: James, R.; Pitts-Singer, T., eds. Bees in agricultural ecosystems. Oxford, UK: Oxford University Press: 48-64.
Cane, J.H.; Love, B.; Swoboda, K. 2012. Breeding biology and bee guild of Douglas’ dustymaiden, Chaenactis douglasii (Asteraceae, Helenieae). Western North American Naturalist. 72(4): 563-568.
Consortium of Pacific Northwest Herbaria [CPNWH]. 2017. Seattle, WA: University of Washington Herbarium, Burke Museum of Natural History and Culture. http://www.pnwherbaria.org/index.php
Cronquist, A.; Holmgren, A.H.; Holmgren, N.H.; Reveal, J.L.; Holmgren, P.K. 1984. Intermountain flora: Vascular plants of the Intermountain West, U.S.A. Vol. 4: Subclass Asteridae (except Asteraceae). New York, NY: The New York Botanical Garden. 573 p.
Eldredge, E.; Novak-Echenique, P.; Heater, T.; Mulder, A.; Jasmine, J. 2013. Plants for pollinator habitat in Nevada. Tech. Note NV 57. Reno, NV: U.S. Department of Agriculture, Natural Resources Conservation Service. 65 p.
European Native Seed Conservation Network [ENSCONET]. 2009. ENSCONET seed collecting manual for wild species. Edition 1: 32 p.
Farr, D.F.; Rossman, A.Y. 2017. Fungal databases, U.S. National Fungus Collections. U.S. Department of Agriculture, Agricultural Research Service. https://nt.ars-grin.gov/fungaldatabases/.
Hammon, B. 2014. Pests affecting native plant seed production. Grand Junction, CO: Colorado State University Extension, Tri-Rivers Area. http://www.wci.colostate.edu/shtml/Lygus.shtml.
Hitchcock, C.L.; Cronquist, A. 2018. Flora of the Pacific Northwest: An illustrated manual. Second Ed. Giblin, D.E.; Legler, B.S.; Zika, P.F.; Olmstead, R.G., eds. Seattle, WA: University of Washington Press. 882 p.
Hitchcock, C.L; Cronquist, A.; Ownbey, M. 1959. Part 4: Ericaceae through Campanulaceae. In: Hitchcock, C.L.; Cronquist, A; Ownbey, M.; Thompson, J.W. Vascular plants of the Pacific Northwest. Seattle, WA: University of Washington Press: 510 p.
Holmgren, N.H. 1979. New Penstemons (Scrophulariaceae) from the Intermountain Region. Brittonia. 31(2): 217-242.
James, D.G.; Nunnallee, D. 2011. Life histories of Cascadia butterflies. Corvallis, OR: Oregon State University Press. 447 p.
Johnston, A. 1970. Blackfoot Indian utilization of the flora of the northwestern Great Plains. Economic Botany. 24(3): 301-324.
Lady Bird Johnson Wildflower Center [LBJWC]. 2020. Penstemon acuminatus Douglas ex. Lindl. Native Plant Database. Austin, TX: Lady Bird Johnson Wildflower Center. https://www.wildflower.org/plants-main [Accessed 2022 April 14].
Martin, A.C.; Zim, H.S.; Nelson, A.L. 1951. American wildlife and plants: A guide to wildlife food habits. New York, NY: Dover Publications. 500 p.
Meyer, S.E.; Kitchen, S.G.; Carlson, S.L. 1995. Seed germination timing patterns in Intermountain Penstemon (Scrophulariaceae). American Journal of Botany. 82(3): 377-389.
Moerman, D. 2003. Native American ethnobotany: A database of foods, drugs, dyes, and fibers of Native American peoples, derived from plants. Dearborn, MI: University of Michigan. http://naeb.brit.org
Nold, R. 1999. Penstemons. Portland, OR: Timber Press. 259 p.
O’Farrell, T.P.; Olson, R.J.; Gilbert, R.O.; Hedlund, J.D. 1975. A population of Great Basin pocket mice, Perognathus parvus, in the shrub-steppe of south-central Washington. Ecological Monographs. 45(1): 1-28.
Ogle, D.; St. John, L.; Stannard, M.; Holzworth, L. 2012. Conservation plant species for the Intermountain West. Plant Materials Technical Note 24. Boise, ID: U.S. Department of Agriculture, Natural Resources Conservation Service. 57 p.
Omernik, J.M. 1987. Ecoregions of the conterminous United States. Map (scale 1:7,500,000). Annals of the Association of American Geographers. 77(1): 118-125.
Parkinson, H. 2003. Landscaping with native plants of the Intermountain Region. Tech. Ref. 1730-3. Boise, ID: U.S. Department of the Interior, Bureau of Land Management. 46 p.
Parkinson, H.; DeBolt, A. 2005. Propagation protocol for production of propagules (seeds, cuttings, poles, etc.) Penstemon acuminatus Douglas ex Lindl. seeds. Native Plant Network. U.S. Department of Agriculture, Forest Service, National Center for Reforestation, Nurseries, and Genetic Resources. http://npn.rngr.net/propagation/protocols [Accessed 2020 June 5].
Pavlik, B.M. 1985. Sand dune flora of the Great Basin and Mojave Deserts of California, Nevada, and Oregon. Madroño. 32(4): 197-213.
Plant Conservation Alliance [PCA]. 2015. National seed strategy for rehabilitation and restoration 2015-2020. Washington, DC: U.S. Department of the Interior, Bureau of Land Management. 52 p.
Plants for a Future [PFAF]. 2020. https://pfaf.org/user/Default.aspx
Richardson, B.; Kilkenny, F.; St. Clair, B.; Stevenson-Molnar, N. 2020. Climate Smart Restoration Tool. https://climaterestorationtool.org/csrt/
Royal Botanic Gardens Kew [RBG Kew]. 2020. Seed Information Database (SID). Version 7.1. http://data.kew.org/sid/ [Accessed 2020 February 16].
Sampangi, R.K.; Almeyda, C.; Druffel, K.L.; Krishna Mohan, S.; Shock, C.C.; Pappu, H.R. 2009. First report of natural infection of Penstemon acuminatus with cucumber mosaic virus in the Treasure Valley of Idaho and Oregon. Plant Disease: An International Journal of Applied Plant Pathology. 93(7): 762.
SEINet – Regional Networks of North American Herbaria Steering Committee [SEINet]. 2017. SEINet Regional Networks of North American Herbaria. Available: https://symbiota.org/seinet/
Shock, C.C.; Feibert, E.B.G.; Rivera, A.; Wieland, K; Shaw, N.; Kilkenny, F. 2019. Native Penstemon species seed yield has little response to irrigation. In: Shock, C.C., ed. Malheur Experiment Station Annual Report 2018. OSU AES Ext/CrS161. Corvallis, OR: Oregon State University: 183-195.
St. John, L.; Ogle, D.; Shaw, N.L. 2009. Plant guide: Sharpleaf penstemon (Penstemon acuminatus Douglas ex. Lindl). Aberdeen, ID: U.S. Department of Agriculture, Natural Resources Conservation Service, Aberdeen Plant Materials Center. 4 p.
Stevens, R.; Jorgensen, K.R.; Young, S.A.; Monsen, S.B. 1996. Forb and shrub seed production guide for Utah. 1996. AG 501. Logan, UT: Utah State University Extension. 52 p.
Taylor, R.J. 1992. Sagebrush country: A wildflower sanctuary. Missoula, MT: Mountain Press Publishing Company. 211 p.
Tilley, D.; Taliga, C.; Burns, C.; St. John, L. 2013. Plant materials for pollinators and other beneficial insects in eastern Utah and western Colorado. Tech. Note 2C. Boise, ID: U.S. Department of Agriculture, Natural Resources Conservation Service. 54 p.
USDA Forest Service, Bend Seed Extractory [USDA FS BSE]. 2017. Nursery Management Information System Version 4.1.11. Local Source Report 34-Source Received. Bend, OR: U.S. Department of Agriculture, Forest Service, Bend Seed Extractory.
USDA Forest Service, Western Wildland Environmental Threat Assessment Center [USFS WWETAC]. 2017. TRM Seed Zone Applications. Prineville, OR: U.S. Department of Agriculture, Forest Service, Western Wildland Environmental Threat Assessment Center. https://www.fs.fed.us/wwetac/threat-map/TRMSeedZoneMapper.php
USDA Natural Resources Conservation Service [USDA NRCS]. 2022. The PLANTS Database. Greensboro, NC: U.S. Department of Agriculture, Natural Resources Conservation Service, National Plant Data Team. https://plants.usda.gov/java.
USDI Bureau of Land Management, Seeds of Success [USDI BLM SOS]. 2017. Seeds of Success collection data. Washington, DC: U.S. Department of the Interior, Bureau of Land Management, Plant Conservation and Restoration Program.
USDI Bureau of Land Management, Seeds of Success [USDI BLM SOS]. 2021. Bureau of Land Management technical protocol for the collection, study, and conservation of seeds from native plant species for Seeds of Success. Washington, DC: U.S. Department of the Interior, Bureau of Land Management, Plant Conservation and Restoration Program. 44 p.
USDI Environmental Protection Agency [USDI EPA]. 2017. Ecoregions. Washington, DC: U.S. Department of the Interior, Environmental Protection Agency. https://www.epa.gov/eco-research/ecoregions
USDI Geological Survey [USDI USGS]. 2017. Biodiversity Information Serving Our Nation (BISON). U.S. Geological Survey. https://bison.usgs.gov/#home
Utah Crop Improvement Association [UCIA]. 2015. How to be a seed connoisseur. Logan, UT: UCIA, Utah Department of Agriculture and Food, Utah State University and Utah State Seed Laboratory. 16 p.
Van Allen Murphey, E. 1990. Indian uses of plants. Glenwood, IL. Meyerbooks. 81 p.
Vankus, V. 2006. Association of official seed certifying agencies (AOSCA). https://www.aosca.org/.
Walker, S.C.; Shaw, N.L. 2005. Current and potential use of broadleaf herbs for reestablishing native communities. In: Shaw, N.L.; Pellant, M.; Monsen, S.B., comps. Sage-grouse habitat restoration symposium proceedings; 2001 June 4-7; Boise, ID. RMRS-P-38. Fort Collins, CO: U.S. Department of Agriculture, Forest Service, Rocky Mountain Research Station: 56-61.
Welsh, S.L.; Atwood, N.D.; Goodrich, S.; Higgins, L.C., eds. 2015. A Utah Flora. Fifth Edition, revised. Provo, UT: Brigham Young University. 990 p.
Young, S.A.; Schrumpf, B.; Amberson, E. 2003. The Association of Official Seed Certifying Agencies (AOSCA) native plant connection. Moline, IL: AOSCA. 9 p. Available: https://seedcert.oregonstate.edu/sites/seedcert.oregonstate.edu/files/pdfs/aoscanativeplantbrochure.pdf
How to Cite
Gucker, Corey L.; Shaw Nancy L. 2022. Sharpleaf penstemon (Penstemon acuminatus Douglas ex Lindl.). In: Gucker, C.L.; Shaw, N.L., eds. Western forbs: Biology, ecology, and use in restoration. Reno, NV: Great Basin Fire Science Exchange. Online: https://westernforbs.org/species/sharpleaf-penstemon-penstemon-acuminatus/