F. Cuticle preparation (abaxial) of A. dim/dia/a: scale bar = 10 )..lm .
Leaf anatomy of the southern African Icacinaceae and its taxonomic significance
by Potgieter M. J., van Wijk A. E. (1999)
M. J. Potgieter* and A. E. van Wijk1
University of Limpopo, Polokwane, South Africa
Department of Botany, University of the North, Private Bag X1106, Sovenga, 0727 Republic of South Africa 1H.G.W J. Schweickerdt Herbarium, Department of Botany, University of Pretoria, 0002 Republic of South Africa
===
in S. Afr. J. Bot. 65(2): 53-62 –
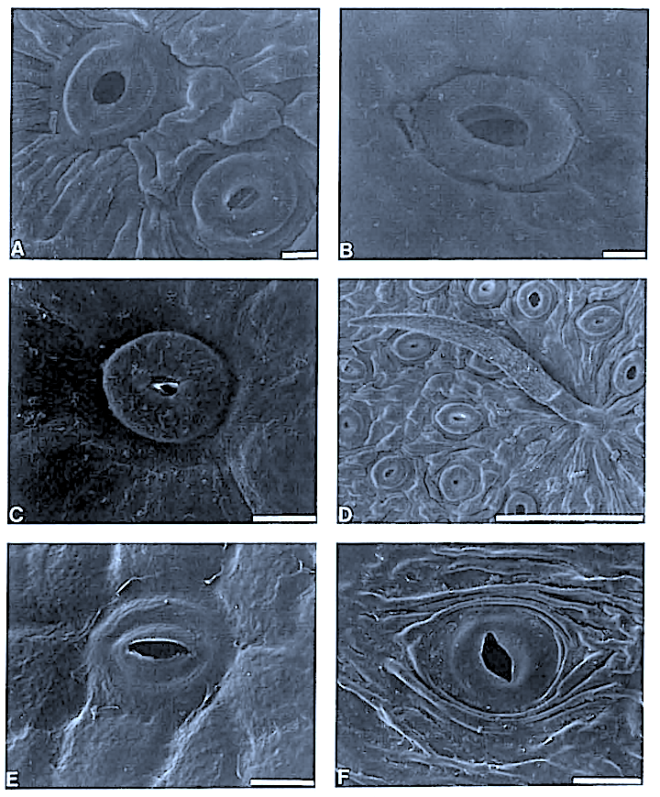
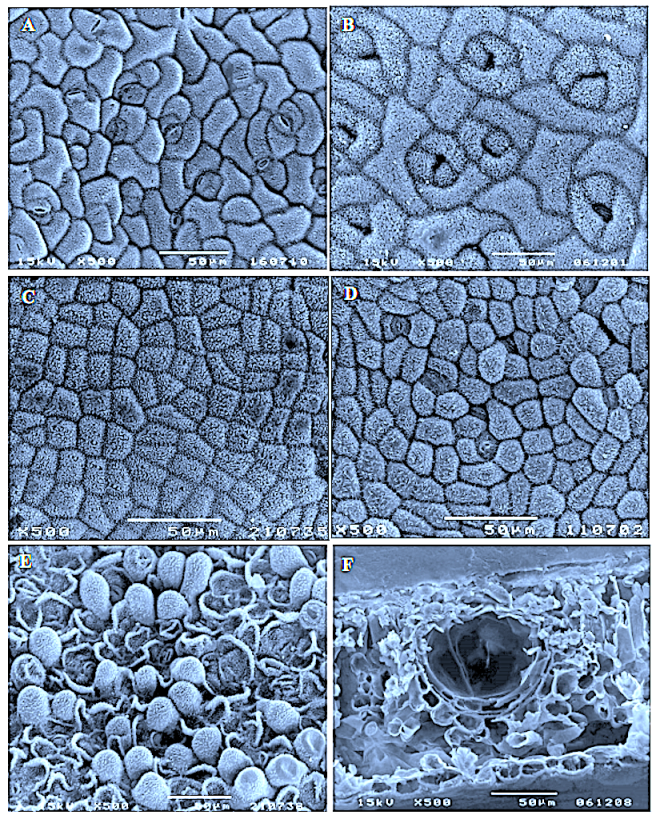
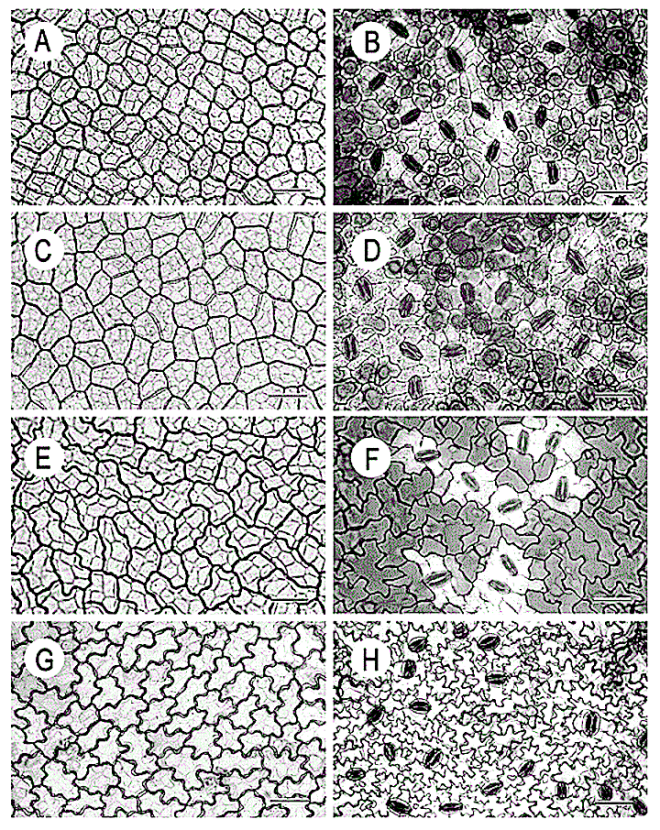
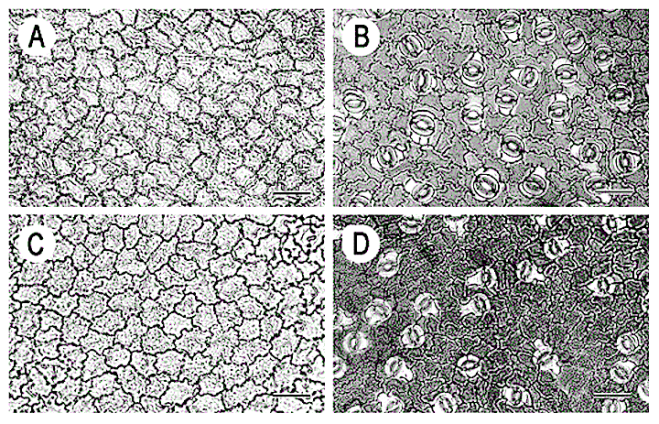
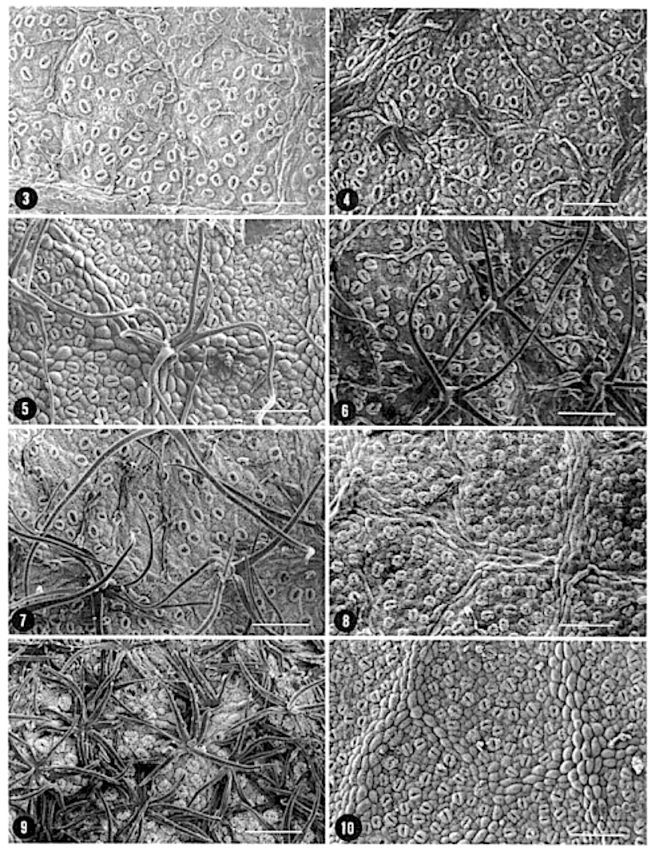
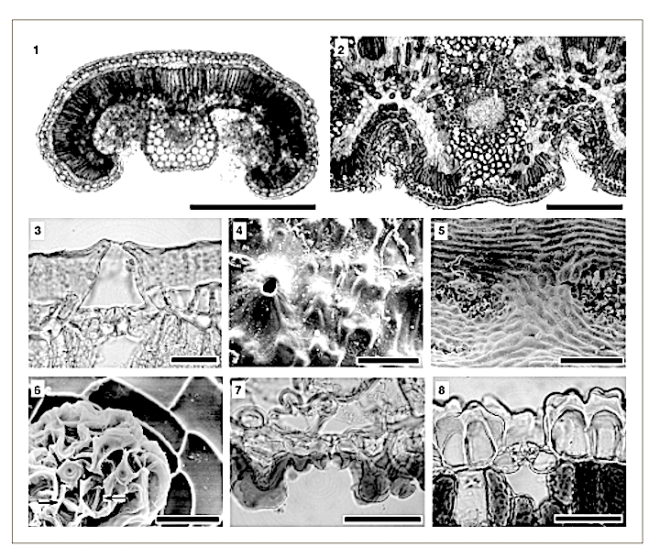
Leaf structure of the three southern African genera of Jcacinaceae was examined by light and scanmng electron microscopy. Diagnostic characters include the stoma and trichome type, and lamina characters, such as mucilage cells, pectic warts and ‘unidentified cell inclusions’,
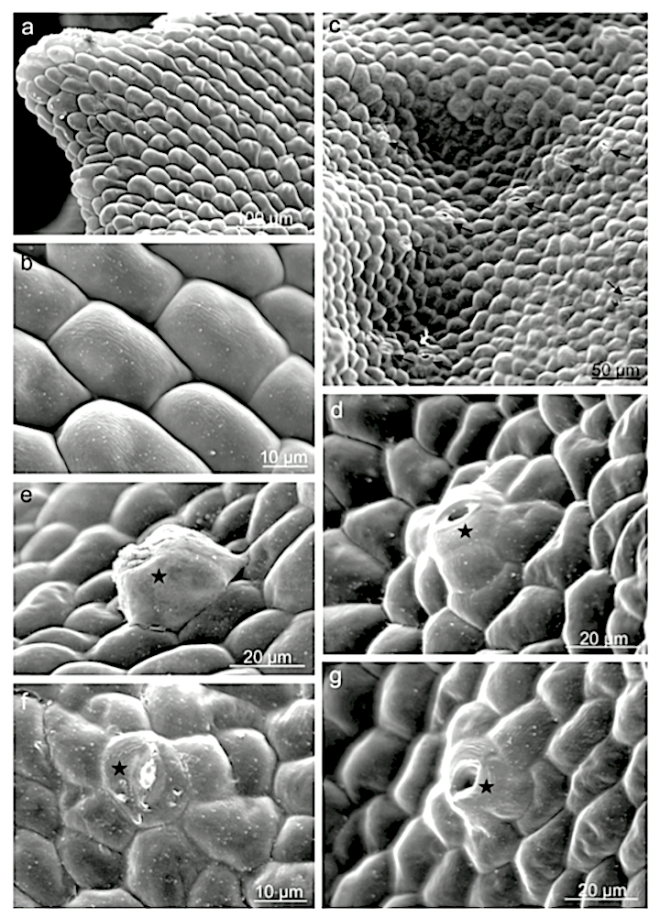
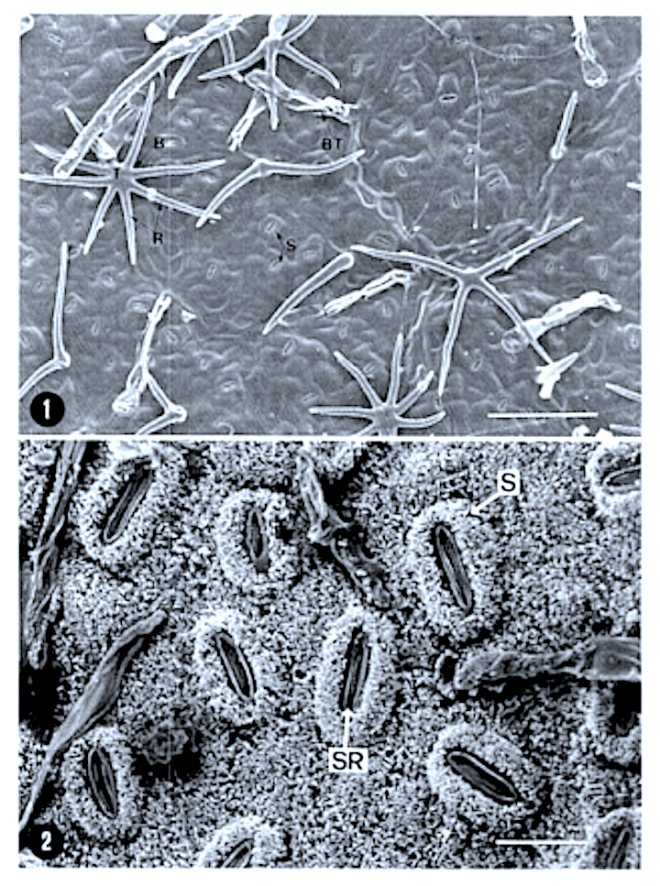
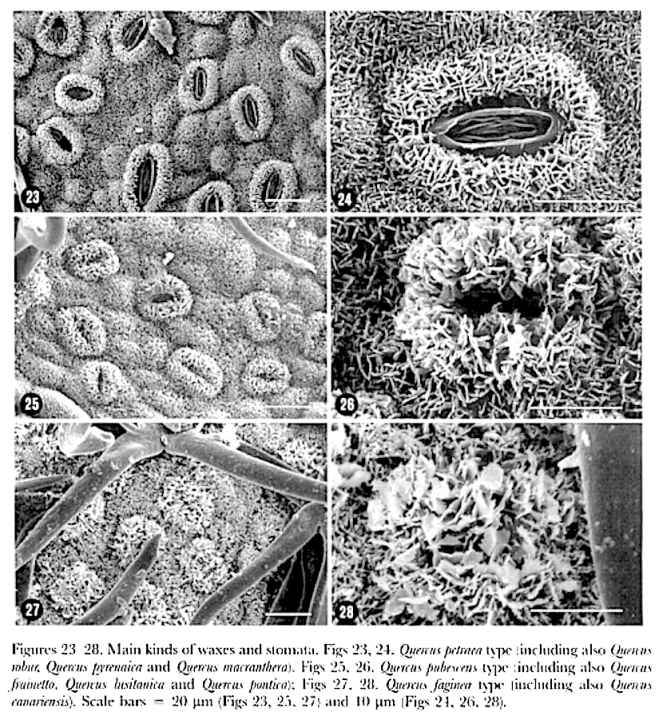
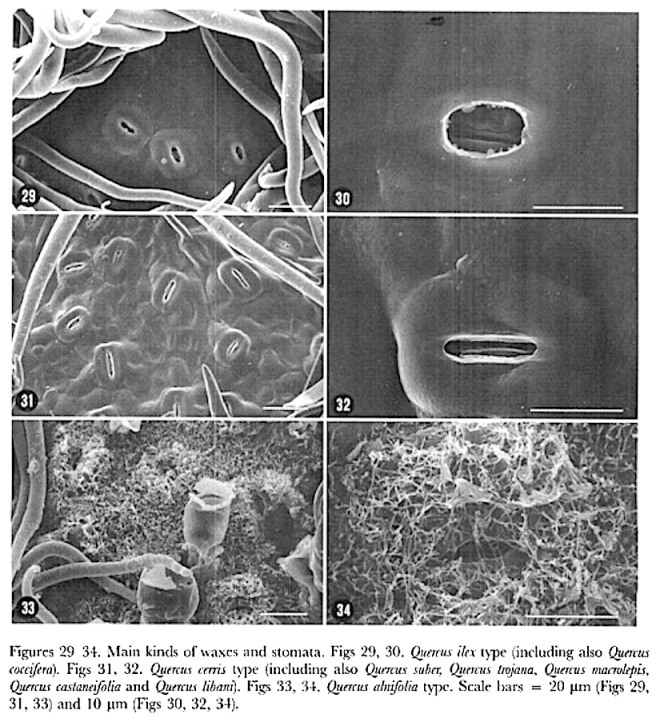
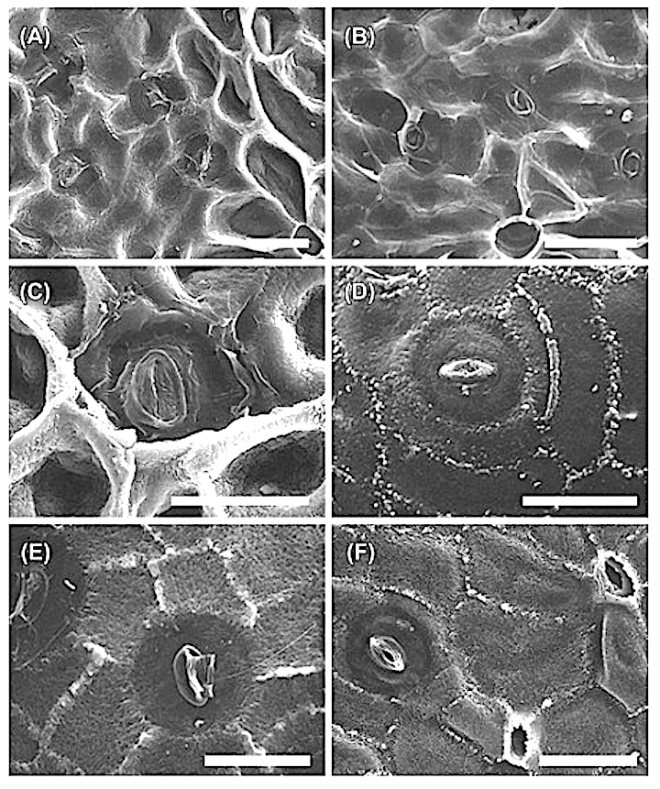
In southern Africa both Cassinopsis Sand. and Pyrenacantha Hook. have cyclocytic stomata, as opposed to the anomocytic type of Apodytes E. Mey. Ex Arn. The presence of a stomatal ridge in A. dimidiata is a useful character in separating this species from the other two southern African members of this genus. A peristomal rim in Cassinopsis tinifolia Harv. and its absence in C. ilicifolia (Hochst.) Kuntze allow the separation of these two species.
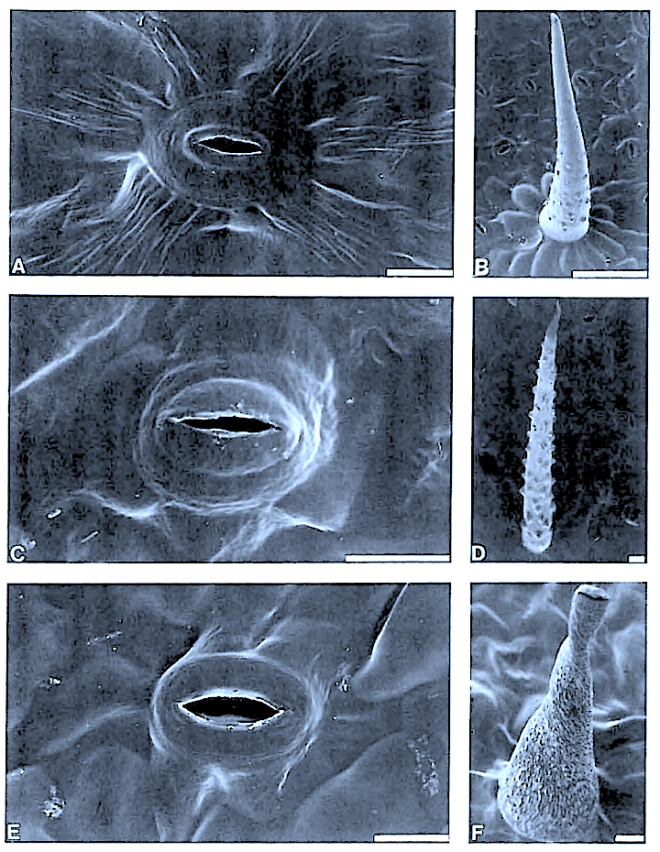
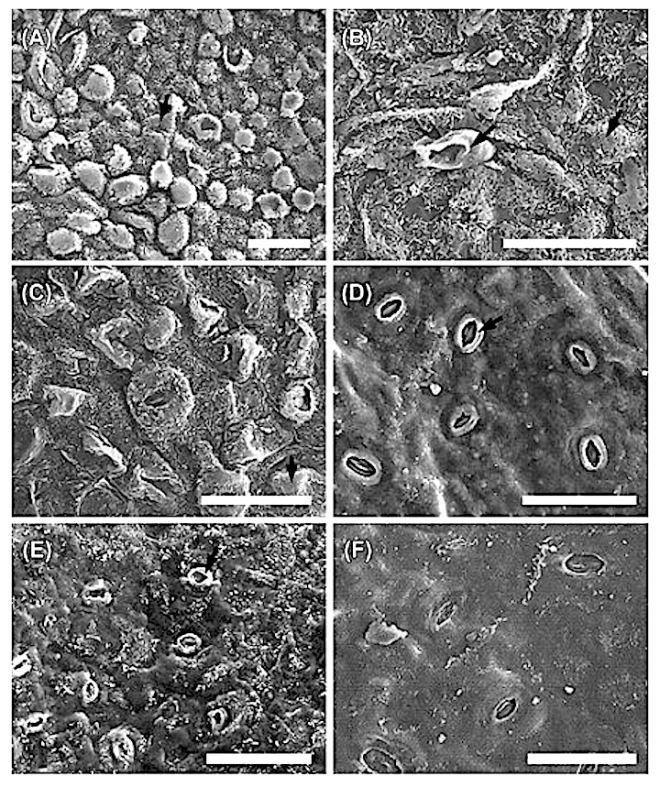
The indumentum of Pyrenacantha consists of simple (unmodified), ‘globular’ and ‘uncinate’ trichomes, whereas that of Apodytes and Cassinopsis consists of simple hairs.
Mucilage cells were found only in members of Apodytes. ln terce!lular, predominantly wart-like pectic protuberances are present in the mesophyll of mature leaf samples of A. ge/denhuysiiVan Wyk & Potgieter and Cassinopsis. Small,
Irregularly shaped , yellowish cell inclusions were found subepidermally to the abaxial epidermis in C. Ificifolia. Their chemical composition and function (if any) is still unknown.
In southern Africa both Cassinopsis and Pyrenacantha have cyclocytic stomata, as opposed to the anomocytic type of Apodytes. According to Van Staveren and Baas (1973) anomocytic stomata are restricted to genera with a low level of specialization, thus representing a primitive condition. Cyclocytic stomata, according to the same authors, occur in genera with a wide range of specialization. Although this type of stomata can be found in genera with a low level of specialization, it is most frequent in highly specialized genera, indicating that this may represent an advanced condition.
Van Staveren and Baas (1 973) found the stomatal index for A. dimidiala to vary between 7 and 21. Research on Celastraceae and Winteraceae also indicates that the stomatal index may be extremely variable within a single species. This invalidates claims by authors in the past that the stomatal index is a diagnostic character at the species level. For this reason we have not considered the stomatal index in this study. In their survey of 26 genera of Maletian lcacinaceae, Van Staveren and Baas (1973) found peristomal rims only in the three genera Codiocarpus Howard, Platea Blume and Stemonurus Blume, indicating that this is a taxonomically useful character state in the Icacinaceae. The presence of a peristomal rim in C. i1iciiolia and its absence in C. tinifolia allows the separation of these two species.

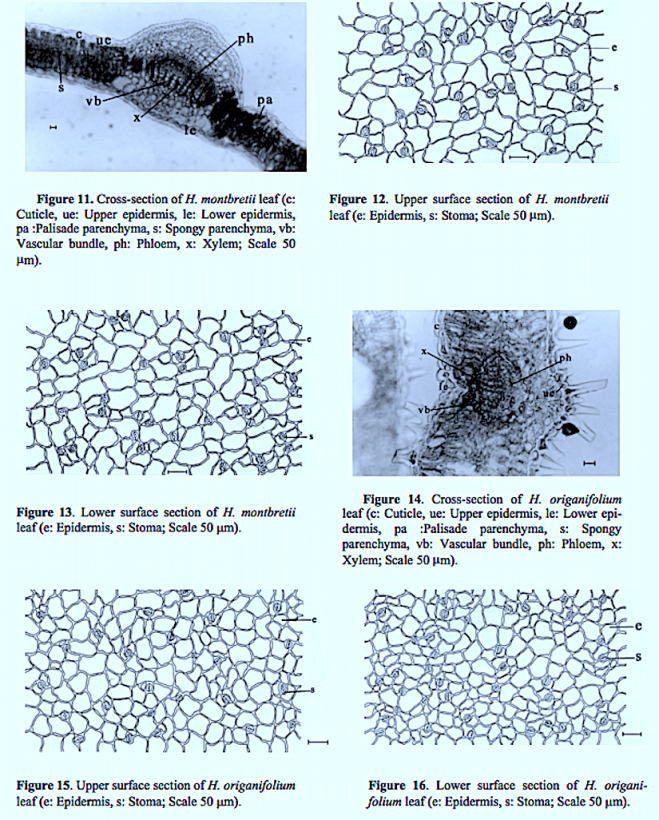




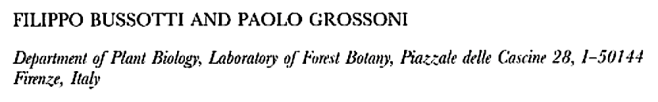



You must be logged in to post a comment.