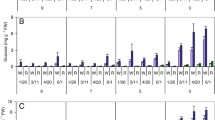
Abstract—In this study, we performed expression analysis of genes associated with cold-induced sweetening in potato tubers: vacuolar invertase (Pain-1), sucrose synthase (SUS4), and invertase inhibitor (InvInh2). Potato varieties Nikulinsky, Symfonia, and Nevsky were used. All three varieties were found to accumulate sugars at low temperatures; the maximum accumulation of reducing sugars was observed at 4°C. It was found that the expression pattern of genes associated with cold-induced sweetening differs depending on the variety and storage duration. The increased expression of vacuolar invertase and its inhibitor is more pronounced at the beginning of storage period, whereas the increased expression of sucrose synthase is more pronounced after 3 months of storage. At early storage periods, high expression of invertase and low expression of inhibitor is observed in the Dutch variety Symfonia, and vice versa in the Russian varieties Nikulinsky and Nevsky. The involvement of the studied genes in the process of cold-induced sweetening is discussed.




Similar content being viewed by others
REFERENCES
Bianchi G., Scalzo R.L., Testoni A., Maestrelli A. 2014. Nondestructive analysis to monitor potato quality during cold storage. J. Food Quality. 37, 9–17.
Zhang H., Hou J., Liu J., Zhang J., Song B., Xie C. 2017. The roles of starch metabolic pathways in the cold-induced sweetening process in potatoes. Starch-Stärke. 69, 1600194.
McCullough M.L., Hodge R.A., Um C.Y., Gapstur S.M. 2019. Dietary acrylamide is not associated with renal cell cancer risk in the CPS-II nutrition cohort. Cancer Epidemiol. Prevention Biomarkers. 28, 616–619.
Sowokinos J.R. 2001. Biochemical and molecular control of cold-induced sweetening in potatoes. Am. J. Potato Res. 78, 221–236.
Amrein T.M., Schönbächler B., Rohner F., Lukac H., Schneider H., Keiser A., Escher F., Amadò R. 2004. Potential for acrylamide formation in potatoes: data from the 2003 harvest. Eur. Food Res. Technol. 219, 572−578.
Chen S., Hajirezaei M.R., Zanor M.I., Hornyik C., Debastn S., Lacomme C., Fernie A.R., Sonnewald U., Boernke F. 2008. RNA interference-mediated repression of sucrose-phosphatase in transgenic potato tubers (Solanum tuberosum) strongly affects the hexose-to-sucrose ratio upon cold storage with only minor effects on total soluble carbohydrate accumulation. Plant Cell Environ. 31, 165–176.
Xiong X., Tai G.C.C., Seabrook J.E.A., Wehling P. 2002. Effectiveness of selection for quality traits during the early stage in the potato breeding population. Plant Breed. 121, 441–444.
Hamernik A.J., Hanneman R.E., Jansky S.H. 2009. Introgression of wild species germplasm with extreme resistance to cold sweetening into the cultivated potato. Crop Sci. 49, 529–542.
Liu X., Zhang C., Ou Y., Lin Y., Song B., Xie C., Liu J., Li X.Q. 2011. Systematic analysis of potato acid invertase genes reveals that a cold-responsive member, Stvac-INV1, regulates cold-induced sweetening of tubers. Mol. Genet. Genom. 286, 109–118.
Bhaskar P.B., Wu L., Busse J.S., Whitty B.R., Hamernik A.J., Jansky S.H., Jiang J. 2010. Suppression of the vacuolar invertase gene prevents cold-induced sweetening in potato. Plant Physiol. 154, 939–948.
Clasen B.M., Stoddard T.J., Luo S., Demorest Z.L., Li J., Cedrone F., Tibebu R., Davison S., Ray E.E., Daulhac A., Coffman A. 2015. Improving cold storage and processing traits in potato through targeted gene knockout. Plant Biotechnol. J. 14, 169–176.
Draffehn A.M., Meller S., Li L., Gebhardt C. 2010. Natural diversity of potato (Solanum tuberosum) invertases. BMC Plant Biol. 10, 1−15.
Slugina M.A., Ryzhova N.N., Kochieva E.Z., Snigir E.A. 2013. Structure and polymorphism of a fragment of the Pain-1 vacuolare invertase locus in Solanum species. Mol. Biol. (Moscow). 47, 215−221. https://doi.org/10.1134/S0026893313020143
Ou Y., Song B., Liu X., Xie C., Li M., Lin Y., Zhang H., Liu J. 2013. Promoter regions of potato vacuolar invertase gene in response to sugars and hormones. Plant Physiol. Biochem. 69, 9–16.
Shumbe L., Visse M., Soares E., Smit I., Dupuis B., Vanderschuren H. 2020. Differential DNA methylation in the Vinv promoter region controls cold induced sweetening in potato. bioRxiv. 062562.
Brummell D.A., Chen R.K., Harris J.C., Zhang H., Hamiaux C., Kralicek A.V., McKenzie M.J. 2011. Induction of vacuolar invertase inhibitor mRNA in potato tubers contributes to cold-induced sweetening resistance and includes spliced hybrid mRNA variants. J. Exp. Bot. 62, 3519–3534.
Liu X., Lin Y., Liu J., Song B., Ou Y., Zhang H., Li M., Xie C. 2013. StInvInh2 as an inhibitor of Stvac INV 1 regulates the cold-induced sweetening of potato tubers by specifically capping vacuolar invertase activity. Plant Biotechnol. J. 11, 640–647.
Liu X., Cheng S., Liu J., Ou Y., Song B., Zhang C., Lin Y., Li X., Xie C. 2013. The potato protease inhibitor gene, St-Inh, plays roles in the cold-induced sweetening of potato tubers by modulating invertase activity. Postharvest Biol. Tech. 86, 265–271.
Baroja-Fernández E., Muñoz F.J., Montero M., Etxeberria E., Sesma M.T., Ovecka M., Bahaji A., Ezquer I., Li J., Prat S., Pozueta-Romero J. 2009. Enhancing sucrose synthase activity in transgenic potato (Solanum tuberosum L.) tubers results in increased levels of starch, ADPglucose and UDPglucose and total yield. Plant Cell Physiol. 50, 1651–1662.
Bagnaresi P., Moschella,A., Beretta O., Vitulli F., Ranalli P., Perata P. 2008. Heterologous microarray experiments allow the identification of the early events associated with potato tuber cold sweetening. BMC Genomics. 9, 1–23.
Baldwin S.J., Dodds K.G., Auvray B., Genet R.A., Macknight R.C., Jacobs J.M.E. 2011. Association mapping of cold-induced sweetening in potato using historical phenotypic data. Ann. Appl. Biol. 158, 248–256.
Liu X., Chen L., Shi W., Xu X., Li Z., Liu T., He Q., Xie C., Nie B., Song B. 2021. Comparative transcriptome reveals distinct starch-sugar interconversion patterns in potato genotypes contrasting for cold-induced sweetening capacity. Food Chem. 334, 127550.
Wiberley-Bradford A.E., Bethke P.C. 2017. Rate of cooling alters chip color, sugar contents, and gene expression profiles in stored potato tubers. Am. J. Potato Res. 94, 534–543.
Slugina M.A., Shchennikova A.V., Meleshin A.A., Kochieva E.Z. 2020. Homologs of vacuolar invertase inhibitor INH2 in tuber-bearing wild potato species and Solanum tuberosum: gene polymorphism and co-expression with saccharolytic enzyme genes in response to cold stress. Sci. Horticult. 269, 109425.
Doroshkov A.V., Simonov A.V., Safonova A.D., Afonnikov D.A., Likhenko I.E., Kolchanov N.A. 2016. Estimation of quantitative characteristics of hairiness of potato leaves using digital microimage analysis. Dostizh. Nauki Tekhn. APK. 30, 12–14.
Alt V.V., Gurova T.A., Elkin O.V., Klimenko D.N., Maksimov L.V., Pestunov I.A., Dubrovskaya O.A., Genaev M.A., Erst T.V., Genaev K.A., Komyshev E.G., Khlestkin V.K., Afonnikov D.A. 2020. The use of Specim IQ, a hyperspectral camera, for plant analysis. Vavilov. Zh. Genet. Sel. 24. 259‒266.
Antonova O.Yu., Shvachko N.A., Novikova L.Yu., Shuvalov O.Yu., Kostina L.I., Klimenko N.S., Shuvalova A.R., Gavrilenko T.A. 2016. Genetic diversity of potato varieties of Russian breeding and CIS countries according to polymorphism of SSR loci and R-resistance gene markers. Vavilov. Zh. Genet. Sel. 20, 596–606.
Totsky I.V., Rozanova I.V., Safonova A.D., Batov A.S., Gureeva Yu.A., Khlestkina E.K., Kochetov A.V. 2021. Genotyping of potato samples from the GenAgro ICG SB RAS collection using DNA markers of genes conferring resistance to phytopathogens. J. Genet. Breed. 25, 677–686.
Khlestkin V.K., Erst T.V., Rozanova I.V., Efimov V.M., Khlestkina E.K. 2020. Genetic loci determining potato starch yield and granule morphology revealed by genome-wide association study (GWAS). Peer. J. 8, e10286.
Khlestkin V.K., Rozanova I.V., Efimov V.M. Khlestkina E.K. 2019. Starch phosphorylation associated SNPs found by genome-wide association studies in the potato (Solanum tuberosum L.). BMC Genet. 20, 45–53.
Ibragimova S., Romanova A., Saboiev I., Salina E., Kochetov A. PlantGen2021: The 6th Int. Sci. Conf. 2021. Novosibirsk, Russia. Abstracts Book. p. 95. Abstract 79.
Turkina M.V., Sokolova S.V. 1971. Methods for the determination of monosaccharides and oligosaccharides. In Biokhimicheskie metody v fiziologii rastenii (Biochemical Methods in Plant Physiology). Moscow: Nauka, pp. 7–34.
Lopez-Pardo R., Ruiz de Galarreta J.I., Ritter E. 2013. Selection of housekeeping genes for qRT-PCR analysis in potato tubers under cold stress. Mol. Breed. 31, 39–45.
Matsuura-Endo C., Ohara-Takada A., Chuda Y., Ono H., Yada H., Yoshida M., Kobayashi A., Tsuda S., Takigawa S., Noda T. 2006. Effects of storage temperature on the contents of sugars and free amino acids in tubers from different potato cultivars and acrylamide in chips. Biosci. Biotechnol. Biochem. 70, 1173–1180.
Lin Q., Xie Y., Guan W., Duan Y., Wang Z., Sun C. 2019. Combined transcriptomic and proteomic analysis of cold stress induced sugar accumulation and heat shock proteins expression during postharvest potato tuber storage. Food Chem. 297, 124991.
Abbasi K.S., Masud T., Qayyum A., Khan S.U., Abbas S., Jenks M.A. 2016. Storage stability of potato variety Lady Rosetta under comparative temperature regimes. Sains Malaysiana. 45, 677–688.
Sonnewald U. 2001. Control of potato tuber sprouting. Trends Plant Sci. 6, 333–335.
Datir S.S., Regan S. 2022. Role of alkaline/neutral invertases in postharvest storage of potato. Postharvest. Biol. Technol. 184, 111779.
Slugina M.A., Kochieva E.Z. 2018. The use of carbohydrate metabolism genes to improve the quality of potato tubers (Solanum tuberosum L.). S-kh. Biol. 53, 450–463.
Gupta S.K., Crants J. 2019. Identification and impact of stable prognostic biochemical markers for cold-induced sweetening resistance on selection efficiency in potato (Solanum tuberosum L.) breeding programs. PLoS One. 14, e0225411.
McKenzie M.J., Sowokinos J.R., Shea I.M., Gupta S.K., Lindlauf R.R., Anderson J.A. 2005. Investigations on the role of acid invertase and UDP-glucose pyrophosphorylase in potato clones with varying resistance to cold-induced sweetening. Am. J. Potato Res. 82, 231–239.
Lin Y., Liu T., Liu J., Liu X., Ou Y., Zhang H., Li M., Sonnewald U., Song B., Xie C. 2015. Subtle regulation of potato acid invertase activity by a protein complex of invertase, invertase inhibitor, and sucrose nonfermenting1-related protein kinase. Plant Physiol. 168, 1807–1819.
Shi W., Ma Q., Yin W., Liu T., Song Y., Chen Y., Song L., Sun H., Hu S., Liu T., Jiang R. 2022. StTINY3 enhances cold-induced sweetening resistance by coordinating starch resynthesis and sucrose hydrolysis in potato. J. Exp. Bot. 73. 4968–4980.
ACKNOWLEDGMENTS
We are grateful to S.V. Gerasimova and D.A. Afonnikov for their help in conducting the study and writing the article.
Funding
The analysis of the sugar content during storage was performed within the framework of the subprogram of the Complex Science and Technology Program “Development of Selection and Seed Production of Potatoes in the Russian Federation,” project no. FWNR-2019-0012. The development of primers and analysis of gene expression was carried out as part of the budgetary project no. FWNR-2022-0017.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no conflicts of interest. This article does not contain any studies involving animals or human participants performed by any of the authors.
Additional information
Translated by M. Batrukova
Rights and permissions
About this article
Cite this article
Egorova, A.A., Saboiev, I.A., Kostina, N.E. et al. Genotype-Specific Features of Cold-Induced Sweetening Process Regulation in Potato Varieties Nikulinsky, Symfonia, and Nevsky. Mol Biol 57, 193–203 (2023). https://doi.org/10.1134/S0026893323020061
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0026893323020061