Abstract
The ‘Nidularioid Complex’ is a group within the Bromelioideae usually characterized by inflorescences nested within water-impounding foliar rosettes. Currently, it comprises six genera: Canistrum, Canistropsis, Edmundoa, Neoregelia, Nidularium, and Wittrockia. While most of these genera occur in eastern Brazil, the distribution of Neoregelia is disjunct between the Atlantic Rainforest and Amazonia. Previous phylogenetic studies have not addressed the monophyly of and relationships among these genera; therefore, we undertook a phylogenetic study of the Nidularioid Complex with emphasis on the genus Neoregelia and its subgenera. A parsimony-based phylogenetic analysis with 101 morphological characters retrieved the Nidularioid Complex as non-monophyletic. Nidularium and Edmundoa were monophyletic. Neoregelia was recovered as non-monophyletic due to the inclusion within it of the Amazonian subgenus Hylaeaicum. These results highlight the need for revision of the generic classification of Bromelioideae, pending increased sampling of taxa and characters.





Similar content being viewed by others
Literature cited
Aguirre-Santoro, J., J. Betancur, G. Brown, T. M. Evans, F. Salgueiro, M. Alves-Ferreira & T. Wendt. 2015. Is Ronnbergia (Bromeliaceae, Bromelioideae) a geographically distinct genus? Evidence from morphology and chloroplast DNA sequence data. Phytotaxa 219: 261–275.
Almeida, V. R., A. F. Costa, A. Mantovani, V. Gonçalves-Esteves, R. C. O. Arruda & R. C. Forzza. 2009. Morphological phylogenetics of Quesnelia (Bromeliaceae-Bromelioideae). Systematic Botany 34: 660–672.
Antonelli, A. & I. Sanmartin. 2011. Why are there so many plant species in the Neotropics? Taxon 60: 403–414.
Baker, J. G. 1889. Handbook of the Bromeliaceae. Georg Bell & Sons, London.
Benzing, D. H. 2000. Bromeliaceae: profile of an adaptive radiation. Cambridge University Press, Cambridge.
B.F.G. (Brazil Flora Group). 2015. Growing knowledge: an overview of seed plant diversity in Brazil. Rodriguésia 66: 1085–1113.
Bremer, K. 1994. Branch support and tree stability. Cladistics 10: 295–304.
Brown, G. K. & E. M. C. Leme. 2000. Dados moleculares em Bromeliaceae. Pp.198–201. In: Leme, E. M. C. (ed.) Nidularium: Bromélias da Mata Atlântica. Salamandra, Rio de Janeiro.
———— & E. M. C. Leme. 2005. The re-establishment of Andrea (Bromeliaceae: Bromelioideae), a monotypic genus from Southeastern Brazil threatened with extinction. Taxon 54: 63–70.
Bukatsch, F. 1972.Bemerkungen zur doppelfarbung astrablau-safranin. Mikrokosmos 6: 225.
Butcher, D. & E. J. Gouda. 2015. The new bromeliad taxon list. University Botanic Gardens, Utrecht. http://BromTaxonList.floraPix.nl (Accessed 31 January 2015).
Chevalier, M. C. 1931. Les Bromeliacées-Nidularinées. Bulletin Mensuel de la Societé Nationale d’Horticulture de France. Series 5: 680–695.
eMonocot. 2010. eMonocot, Version 1.0.2. http://e-monocot.org (Accessed 20 December 2014).
Evans, T., R. S. Jabaily, A. P. G. Faria, L. O. F. Sousa, T. Wendt & G. K. Brown. V2015. Phylogenetic relationships in Bromeliaceae subfamily Bromelioideae based on chloroplast DNA sequence data. Systematic Botany 40: 116–128.
Givnish, T. J., K. C. Millam, P. E. Berry & K. J. Sytsma. 2007. Phylogeny, adaptive radiation and historical biogeography of Bromeliaceae inferred from ndhF sequence data. Aliso 23: 3–26.
————, M. H. J. Barfuss, B. Van Ee, R. Riina, K. Schulte, R. Horres, P. A. Gonsiska, R. S. Jabaily, D. M. Crayn, J. A. C. Smith, K. Winter, G. K. Brown, T. M. Evans, B. K. Holst, H. Luther, W. Till, G. Zizka, P. E. Berry & K. J. Sytsma. 2011. Phylogeny, adaptive radiation, and historical biogeography in Bromeliaceae: insights from an eight-locus plastid phylogeny. American Journal of Botany 98: 872–895.
Halbritter, H. 1998. Preparing living pollen material for scanning electron microscopy using 2, 2-dimethoxypropane (DMP) and critical-point drying. Biotechnic Histochemistry 73: 137–143.
Hallbritter H.M. & W. Till. 1998. Morfologia polínica do complexo nidularióide. Pp 114–121. In: E. M. C. Leme (ed). Canistropsis: Bromélias da Mata Atlântica. Salamandra, Rio de Janeiro.
Heller, S., E. M. C. Leme, K. Schulte, A. M. Benko-Iseppon & G. Zizka. 2015. Elucidating relationships in the Aechmea alliance: AFLP analysis of Portea and the Gravisia complex. Systematic Botany 40: 716–725.
Hesse M., H. Halbritter, R. Zetter, M. Weber, R. Buchner, A. Frosch-Radivo & S. Ulrich. 2009. Pollen terminology. Springer, Wien.
Horres, R., K. Schulte, K. Weising & G. Zizka. 2007. Systematics of Bromelioideae (Bromeliaceae)—Evidence from molecular and anatomical studies. Aliso 23: 27–43.
Leme, E. M. C. 1997. Canistrum: Bromélias da Mata Atlântica. Salamandra. Rio de Janeiro.
————. 1998. Canistropsis: Bromélias da Mata Atlântica. Salamandra. Rio de Janeiro.
————. 2000. Nidularium: Bromeliads of the Atlantic Forest. Hamburg Donneley Editora Gráfica, Rio de Janeiro.
————, W. Till, G. K. Brown, J. R. Grant & R. H. A. Govaerts. 2008. Eduandrea, a new generic name for Andrea. Journal of the Bromeliad Society 58: 61–64.
Luther H. E. 1989. Neoregelia johnsoniae, an extraordinary new species from Eastern Peru. Journal of Bromeliad Society 39(8): 70–71.
————. 2012. An alphabetical list of bromeliad binomials. Bromeliad Society International, Sarasota, Florida.
Maddison, W. P. & D. R. Maddison. 2011. Mesquite: a modular system for evolutionary analysis. Version 2.75. http://mesquiteproject.org
Mantovani, A. 2002. Alocação reprodutiva, germinação de sementes e estabelecimento de três bromélias terrestres de restinga. Ph.D. Thesis. Universidade Federal do Rio de Janeiro.
———— & R. R. Iglesias. 2009. Size-dependent allocation of biomass to ancillary versus flowers of the inflorescences of the epiphyte Tillandsia stricta Soland (Bromeliaceae). Acta Botanica Brasilica 23: 130–135.
Martinelli, G., C. M. Vieira, M. Gonzalez, P. Leitman, A. Piratininga, A. F. Costa & R. C. Forzza. 2008. Bromeliaceae da Mata Atlântica brasileira: lista de espécies, distribuição e conservação. Rodriguésia. 59: 209–258.
Mez, C. 1894. Bromeliaceae. Pp.173–643. In: Von Martius, C. P. P., A. Eichler & I. Urban (eds.), Flora Brasiliensis, vol. 3(3), Muchen, Wien, Leipzig.
————. 1896. Bromeliaceae. In: C. De Candolle (ed). Monographiae phanerogamarum prodromi nunc continuatio, nunc revisio, vol. 9. G. Masson, Paris.
Monteiro, R. F., R. C. Forzza & A. Mantovani. 2011. Leaf structure of Bromelia and its significance for the evolution of Bromelioideae (Bromeliaceae). Plant Systematics and Evolution 293: 53–64.
Nixon, K. C. 2002.Winclada, Version 1.00.08. Published by the author. http://www.cladistics.com/wincDownload.htm.
Nogueira, F.M., Fagundes, N. F., Kuhn, S.A., J. N. Fregonezi & J. E. A. Mariath 2015. Ovary and ovule anatomy in the nidularioid complex and its taxonomic utility (Bromelioideae: Bromeliaceae). Botanical Journal of the Linnean Society 177(1):66-77.
O`Brien, T. P. & M. E. McCully. 1981. The study of plant structure: principles and selected methods. Thermarcarphi, Melbourne.
Radford, A. E., W. C. Dickinson, J. R. Massey & C. R. Bell. 1974. Vascular plant systematics. Harper and Row, New York.
Ramírez, I. M. 1991. Systematic revision of Neoregelia subgenus Hylaeaicum (Bromeliaceae). Master’s Thesis, University of Missouri-St. Louis.
Ramírez, I. M. 2000. Neoregelia subgenus Hylaeaicum. Pp 545–550. In: D. H. Benzing (ed.) Bromeliaceae: Profile of an adaptive radiation. Cambrigde University Press, Cambridge, Massachusetts.
Safford, H. D. 1999. Brazilian Páramos I. An introduction to the physical environment and vegetation of the campos de altitude. Journal of Biogeography 26: 693–712.
Sajo, M. G.; S. R. Machado & S. M. Carmello-Guerreiro. 1998. Aspectos estruturais de folhas de bromélia e suas implicações no agrupamento de espécies. Pp. 102–111. In: E. M. C. Leme. Canistropsis: Bromélias da Mata Atlântica. Salamandra, Rio de Janeiro.
Sass, C. & C. D. Specht. 2010. Phylogenetic estimation of the core bromelioids with emphasis on the genus Aechmea (Bromeliaceae). Molecular Phylogenetics and Evolution 55: 559–571.
Schulte, K., R. Horres & G. Zizka. 2005. Molecular phylogeny of Bromelioideae and its implications on biogeography and evolution of CAM in the family (Poales, Bromeliaceae). Senckengergiana Biologica 85: 113–125.
————, M. H. J. Barfuss & G. Zizka. 2009. Phylogeny of Bromelioideae (Bromeliaceae) inferred from nuclear and plastid DNA loci reveals the evolution of the tank habit within the subfamily. Molecular Phylogenetics and Evolution 51; 327–339.
Silvestro, D., G. Zizka & K. Schulte. 2014. Disentangling the effects of key innovations on the diversification of Bromelioideae (Bromeliaceae). Evolution 68: 163–175.
Smith, L. B. 1955.The Bromeliaceae of Brazil. Smithsonian Miscellaneous Collections 126(1): 1–290.
———— & R. J. Downs. 1979. Bromelioideae (Bromeliaceae). Flora Neotropica Monograph 14. The New York Botanical Garden, New York.
Swofford, D. L. 2004. PAUP*4.0: Phylogenetic analysis using parsimony (*and other methods). Version 4.0b10. Sinauer Associates, Sunderland, Massachusetts.
Tomlinson, P. B. 1969. Commelinales-Zingiberales, Pp. 193–294 In: C.R. Metcalfe (ed.), Anatomy of the monocotyledons. Claredon Press, Oxford.
Weberling, F. 1989. Morphology of flowers and inflorescences. Cambridge University Press, Cambridge.
Acknowledgments
This work is part of the MSc. dissertation of AKLV, developed at the Jardim Botânico do Rio de Janeiro. FSS is grateful to CAPES for a doctoral scholarship (Edital MCT/CNPq/MEC/Capes No 52/2010–PROTAX). The authors thank Gustavo Martinelli for providing plant material, Monique F. Neves for assistance in preparing leaf sections, Daniela Zappi for help with content and English writing, and Julián Aguirre-Santoro and an anonymous reviewer for their critics and suggestions that greatly improved the manuscript. RCF is a CNPq research fellow.
Author information
Authors and Affiliations
Corresponding authors
Electronic supplementary material
Below is the link to the electronic supplementary material.
Suppl. Material 1
Inflorescence types present in the studied specimens with voucher numbers. A. Neoregelia coimbrae, corymb (RB306979). B. Quesnelia arvensis, spadix (RB338965). C. Canistrum aurantiacum, capitulum (RB330755). D. Aechmea mertensii, spike (RB201814). E. Neoregelia longisepala, closed panicule (corymbform) (RB536616). F. Aechmea echinata, umbel of capitula (RB420012). G. Canistropsis billbergioides, raceme of capitula (RB430452). H. Aechmea caesia, double spike (RB587706). (GIF 255 kb)
ESM 1
(DOCX 33 kb)
Suppl. Material 3
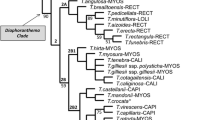
Accelerated transformation (ACCTRAN) character optimization in one of the most parsimonious trees (MPT 300) of the Nidularioid Complex morphological analysis. Character symbols are shaded as follows: white circles=homoplastic, black circles=non-homoplastic. Numbers above the circles represent character numbers, and those below the circles represent character states. The different subgenera of Neoregelia are coded as follows: black circle=Neoregelia subg. Neoregelia, white square=Neoregelia subg. Longipetalopsis, white triangle=Neoregelia subg. Protoregelia, gray hexagon=Neoregelia subg. Hylaeaicum. (GIF 58 kb)
Appendices
Appendix 1. Specimens used in this study.
Aechmea – A. caesia E. Morren ex Baker: Costa 316 (RB); Martinelli 13365, 10709 (RB); Silva 802 (RB); Wendt 168 (RB). A. capixabae L. B. Sm.: Marquete 3894 (RB); Martinelli 11621, 15640 (RB); Silva 1094 (RB); Vieira 971 (RB). A. echinata (Leme) Leme: Martinelli 15434 (RB). A. mertensii (G. Mey.) Schult. & Schult. f.: Duarte 7033 (RB); Farney 1934 (RB); Forzza 289, 5951 (RB); Pereira 794 (RB); Quinet 1980, 1983 (RB). A. mollis L.B.Sm. Jardim 4840 (RB); A. ramosa Mart. ex Schult. & Schult. f.: Forzza 5251 (RB); Jardim 3157 (RB); Wendt 159 (RB). A. weberi (E. Pereira & Leme) Leme: Martinelli 15419 (RB). Billbergia – B. amoena Lindl.: Almeida 45 (RB), Costa 6 (RB), Forzza 2405 (RB). Bromelia – B. minima Leme & Esteves: Martinelli 16227 (RB); Silva 1253 (RB). B. serra Griseb.: Forzza 2320, 3762, 3765 (RB); Monteiro 232 (RB). Canistropsis – C. billbergioides (Schult. & Schult. f.) Leme: Moreira 223 (RB); C. burchelli (Baker) Leme: Moreira 210 (RB). Canistrum – C. alagoanum Leme & J. A. Siqueira: Martinelli 15337 (RB). C. aurantiacum Morren: Martinelli 15130 (RB). C. guzmanioides Leme: Martinelli 15367 (RB). Edmundoa – E. lindenii (Regel) Leme: Forzza 4465 (RB); E. perplexa (L. B. Sm.) Leme: Martinelli 15939 (RB); Hohenbergia – H. blanchetii E. Morren ex Baker: Silva 61 (RB), Foster 75 (R). Neoregelia subg. Neoregelia – N. carolinae (Beer) L. B. Sm: Martinelli 15508 (RB). N. cathcartii C. F. Reed & Read: Leme 2545 (RBvb live collection JBRJ). N. chlorosticta (Baker) L. B. Sm.: Venda s.n. (RB 506197). N. coimbrae E. Pereira & Leme: H. Lima 6484 (RB). N. concentrica (Vell.) L. B. Sm: Martinelli 1716 (RB). N. crispata Leme: Martinelli 15403 (RB). N. cruenta (R. Graham) L. B. Sm.: Venda s.n. (RB 515588). N. dungsiana E. Pereira: Venda s.n. (RB 515584). N. farinosa (Ule) L. B. Sm.: Faria s.n. (RB 504194). N. johannis (Carrière) L. B. Sm.: Reis 1043 (RB). N. johnsoniae H. Luther: Leme 2568 (RBvb live collection JBRJ). N. kautskyi E. Pereira: Martinelli 15643 (RB). N. laevis (Mez) L. B. Sm.: Martinelli 15859 (RB). N. leprosa L. B. Sm. Martinelli 15709 (RB). N. liliputiana E. Pereira: Amaral s.n. (RBvb 1118). N. lymaniana R. Braga & Sucre: Venda 14 (BHCB, RB). N. macahensis (Ule) L. B. Sm. Martinelli s.n. (RB 508260). N. sarmentosa (Regel) L. B. Sm.: Bocayuva 98 (RB). N. cf. smithii W. Weber: Venda s.n. (RBvb live collection JBRJ). N. spectabilis (T. Moore) L. B. Sm.: Dias-Mello 997 (RB). N. zonata L. B. Sm: Foster 197 (RB). Neoregelia subg. Longipetalopsis Leme–N. bahiana (Ule) L. B. Sm.: Jardim 2553 (RB); Martinelli 2683, 4377, 5527, 11259 (RB). N. diversifolia E. Pereira: Forzza 4981 (RB); Martinelli 15751 (RB). N. ibitipocensis (Leme) Leme: Forzza 3338, 3992 (RB); Martinelli 15317 (RB). N. kerryi Leme: Venda s.n. (RB 515592). N. leucophoea (Baker) L. B. Sm.: Costa 508 (RB); Leme 1107 (HB, RB). N. longipedicellata Leme: Martinelli 16007 (RB). N. mucugensis Leme: Brito 2097 (RB). N. paulistana E. Pereira: Seidel 1078 (RB). N. tenebrosa Leme: Costa s.n. (RB 326175). Neoregelia subg. Protoregelia Leme–N. longisepala E. Pereira & I. A. Penna: Leme 3049 (RB). Neoregelia subg. Hylaeaicum L. B. Sm.–N. eleutheropetala (Ule) L. B. Sm.: Hopkins 1437 (RB); Matteo 109 (RB). N. aff. leviana L. B. Sm.: P. Carauta (RB 186108), Leme 2777 (RBvb 1782, live collection JBRJ) N. margaretae L. B. Sm.: Venda s.n. (E. Leme, private collection, RBvb live collection JBRJ). N. myrmecophila (Ule ex G. Karsten & H. Schenk) L. B. Sm.: Costa 194 (RB). N. tarapotoensis Rauh: Luther s.n. (E. Leme, private collection, RBvb 1780, live collection JBRJ). Nidularium–N. innocentii Leme: Martinelli 15387 (RB). N. itatiaiae L. B. Sm.: Fonseca 3 (RB). N. kautskyanum Leme: Martinelli 15685 (RB). N. meeanum Leme: Moreira 226 (RB). N. utriculosum Ule: Lima 6487 (RB). Portea – P. alatisepala Philcox: Almeida 47 (RB), Kautsky 236 (RB), Martinelli 15414 (RB). Quesnelia–Q. arvensis (Vell) Mez: Forzza 4833 (RB); Martinelli 7789 (RB); Vieira 1202, 1234 (RB). Q. humilis Mez: Ferreira 506 (RB); Leme 3473 (RB); Vieira 950 (RB). Wittrockia–W. cyathiformis (Vell) Leme: Brade 21150 (RB); Heiden 892 (RB); Oliveira 1115 (RB); Sucre 5193 (RB). W. gigantea (Baker) Leme: Forzza 3139, 3600, 4192 (RB); Kolmann 6747, 7585 (RB); Monteiro 29 (RB). W. superba Lindm. Fontoura 161 (RB); Silva 145 (RB).
Appendix 2. Characters and character states used in the cladistic analysis of the Nidularioid Complex.
1. Leaves, phyllotaxy, tank: 0=absent; 1=present. 2. Leaves, phyllotaxy, rosette, general shape (live plants/herbarium specimens): 0=broadly infudibuliform; 1=narrowly infundibuliform 2=tubulose. 3. Leaves, blade, shape: 0=linear; 1=linear-triangular; 2=narrowly oblong; 3=narrowly elliptic; 4=oblanceolate; 5=lanceolate; 6=oblong. 4. Leaves, apex, shape (excluding the projection): 0=attenuate; 1=acute; 2=obtuse; 3=emarginate; 4=rounded. 5. Leaves, apex, projection, shape: 0=absent; 1=apiculate; 2=pungent. 6. Leaves, margin, type: 0=entire; 1=aculeate. 7. Leaves, margin, spines, size: 0=conspicuous; 1=inconspicuous. 8. Leaves, margin, spines, orientation (in the same leaf): 0=antrorse; 1=antrorse and retrorse. 9. Leaves, margin, spines, color: 0=brown; 1=green; 2=red; 3=vinaceous. 10. Leaves, sheath transition to blade: 0=abrupt; 1=tenuous. 11. Leaves, sterile blade, color: 0=green; 1=green with vinaceous or reddish maculae; 2=green with a reddish macula at the apex; 3=completely red. 12. Central leaves, color change during anthesis: 0=absent; 1=present. 13. Leaves, sheath, trichomes: 0=lepidote; 1=pannose. 14. Inflorescence, peduncle, size compared to rosette: 0=included; 1=exceeding the rosette. 15. Inflorescence, peduncle, trichomes: 0=absent; 1=present. 16. Inflorescence, peduncle, trichome, type: 0=velutinous; 1=tomentose; 2=floccose; 3=villous; 4=lepidote; 5=lanuginose. 17. Inflorescence, peduncle, bracts, cover type: 0=completely covered, imbricate; 1=partially covered, lax. 18. Inflorescence, peduncle, color at anthesis: 0=white; 1=red; 2=green; 3=brown. 19. Inflorescence, peduncle, bracts, shape: 0=ovate; 1=oblong; 2=elliptic; 3=lanceolate; 4=linear; 5=widely ovate; 6=narrowly ovate; 7=narrowly elliptic; 8=depressed ovate. 20. Inflorescence, peduncle, bract, apex, shape: 0=attenuate; 1=acuminate; 2=rounded; 3=caudate. 21. Inflorescence, peduncle, bract, apex projection, shape: 0=absent; 1=apiculate; 2=pungent; 3=caudate. 22. Inflorescence, peduncle, bract, trichomes: 0=absent; 1=present. 23. Inflorescence, peduncle, bract, trichome, type: 0=lepidote; 1=tomentose; 2=floccose; 3=villous; 4=lanuginose. 24. Inflorescence, peduncle, bract, margin, type: 0=entire; 1=aculeate. 25. Inflorescence, peduncle, bract, color: 0=stramineous with a red apex; 1=green; 2=vinaceous; 3=white; 4=red; 5=pinkish; 6=brown. 26. Inflorescence, structure: 0=simple; 1=compound. 27. Inflorescence, type: 0=corymb; 1=spadix; 2=capitulum; 3=spike; 4=closed panicule (corymbiform); 5=umbel of capitula; 6=raceme of capitula; 7=double spike. 28. Inflorescence, anthesis, order of flower anthesis: 0=centripetal; 1=unordered; 2=centrifugal. 29. Inflorescence, floral bracts, size: 0=exceeding/equaling the pedicel; 1=exceeding/equaling the ovary; 2=exceeding/equaling the sepal; 3=exceeding/equaling the petal. 30. Inflorescence, floral bracts, color: 0=white; 1=stramineous; 2=red; 3=pinkish; 4=brown; 5=green; 6=yellow. 31. Inflorescence, floral bracts, shape: 0=ovate; 1=oblanceolate; 2=oblong; 3=lanceolate; 4=linear; 5=narrowly elliptic; 6=ovate; 7=narrowly-triangular; 8=elliptic; 9=narrowly-oblong. 32. Inflorescence, floral bracts, margin, type: 0=entire; 1=aculeate. 33. Inflorescence, floral bracts, trichome: 0=absent; 1=present; 34. Inflorescence, floral bracts, trichome, type: 0=lepidote; 1=tomentose; 2=floccose; 3=villous; 4=lanuginose. 35. Inflorescence, floral bracts, trichome, location: 0=apex; 1=whole bract. 36. Inflorescence, floral bracts, keel: 0=absent; 1=present. 37. Inflorescence, floral bracts, apex, shape: 0=acute; 1=attenuate; 2=obtuse; 3=rounded. 38. Inflorescence, floral bracts, apex projection: 0=absent; 1=acuminate; 2=apiculate; 3=pungent. 39. Flower, sepal, symmetry: 0=symmetric; 1=subsymmetric to asymmetric. 40. Flower, sepal, shape: 0=ovate; 1=obovate; 2=lanceolate; 3=linear; 4=oblanceolate. 41. Flower, sepal, color: 0=white; 1=brown; 2=red; 3=green; 4=pinkish; 5=yellow; 6=vinaceous; 7=stramineous. 42. Flower, sepal, trichome: 0=absent; 1=present. 43. Flower, sepal, trichome, type: 0=lepidote; 1=tomentose; 2=floccose; 3=villous; 4=lanuginose. 44. Flower, sepal, trichome, location: 0=apex; 1=whole sepal; 2=base. 45. Flower, sepal, apex, shape: 0=asymmetric; 1=acute; 2=attenuate; 3=rounded; 4=apiculate. 46. Flower, sepal, apex projection, shape: 0=absent; 1=acute; 2=apiculate; 3=caudate; 4=pungent. 47. Flower, sepal, consistency: 0=membranaceous; 1=cartaceous; 2=coriaceous. 48. Flower, sepal, keel: 0=absent; 1=present. 49. Flower, sepal, margin, type: 0=entire; 1=aculeate. 50. Flower, sepal, fusion: 0=free; 1=connate. 51. Flower, sepal, fusion, degree: 0=completely connate; 1=connate only at base. 52. Flower, pedicel: 0=indistinct or absent 1=distinct. 53. Flower, petal, consistency: 0=membranous; 1=cartaceous. 54. Flower, petal, apex blade, color distinction: 0=similar to the blade; 1=distinct to the blade. 55. Flower, petal, blade, color: 0=white; 1=pinkish; 2=purple; 3=green; 4=blue; 5=yellow. 56. Flower, petal, apex, color: 0=lilac; 1=red; 2=purple; 3=pinkish; 4=blue; 5=white; 6=yellow; 7=green; 8=orange. 57. Flower, petal, color, distinction between apex and its margins: 0=margins distinct to the apex; 1=margins equal to the apex. 58. Flower, petal, apex, shape: 0=acute; 1=obtuse; 2=rounded. 59. Flower, petal, position at anthesis: 0=suberect to erect; 1=patent to reflexed; 2=curved inwards. 60. Flower, petal, shape: 0=oblanceolate; 1=oblong; 2=obovate; 3=elliptic; 4=linear; 5=triangular; 6=narrowly obovate; 7=lanceolate; 8=narrowly oblong; 9=narrowly elliptic. 61. Flower, petal, fusion: 0=free; 1=connate. 62. Flower, petal, fusion, degree: 0=connate at base; 1=connate up to 1/3 of the length; 2=connate more than 1/3 of the length. 63. Flower, petal, size: 0=up to 5 cm; 1=upwards from 6 cm. 64. Flower, petal, cucullate apex: 0=absent; 1=present. 65. Flower, petal, petal appendages: 0=absent; 1=present. 66. Flower, petal, appendage, shape: 0=spatulate; 1=fimbriate; 2=cupuliform. 67. Flower, petal, callosity: 0=absent; 1=present. 68. Flower, petal, trichomes: 0=absent; 1=present. 69. Androecium, anther, color at anthesis: 0=white; 1=yellow. 70. Androecium, adnation to petals: 0=adnate; 1=free; 2=three antipetal adnate and three free. 71. Androecium, anther, shape: 0=oblong; 1=sagittate; 2=elliptic; 3=linear; 4=obtuse. 72. Gynoecium, stigma, shape: 0=equally long and wide; 1=longer than wide. 73. Gynoecium, ovary, shape: 0=ovate; 1=elliptic; 2=oblong; 3=obovate; 4=subcylindrical; 5=pyriform. 74. Gynoecium, ovary, furrow: 0=absent; 1=present. 75. Gynoecium, ovary, ovules location along ovary wall without considering type of placentation: 0=at apex; 1=up to half the length; 2=only at half. 76. Anatomy, leaf, adaxial surface, contour 0=smooth to slightly wavy; 1=groovy. 77. Anatomy, leaf, abaxial surface, contour: 0=smooth to slightly wavy; 1=groovy. 78. Anatomy, leaf, epidermis, adaxial surface, shape and thickness of epidermal cells: 0=internal periclinal wall highly thickened, reduced lumen; 1=internal periclinal wall poorly thickened, wide lumen. 79. Anatomy, leaf, epidermis, abaxial surface, shape and thickness of epidermal cells: 0=internal periclinal wall strongly thickened, reduced lumen; 1=internal periclinal wall weakly thickened, wide lumen. 80. Anatomy, leaf, epidermis, scale, pedicle, number of cells: 0=two; 1=more than two. 81. Anatomy, leaf, epidermis, stomata, position compared to remaining epidermal cells: 0=same level; 1=under. 82. Anatomy, leaf, mesophyll, adaxial surface, mechanical hypodermis, thickened cells: 0=absent; 1=with one thickened layer and one or more transition layers; 2=more than one highly thickened layer. 83. Anatomy, leaf, mesophyll, abaxial surface, mechanical hypodermis, thickened cells: 0=absent; 1=with one thickened layer and one or more transition layers; 2=two or more strongly thickened layers. 84. Anatomy, leaf, mesophyll, aquiferous hypodermis, extension: 0=up to the thickness of 1/3 of the leaf blade; 1=more than the thickness of 1/3 of the leaf blade. 85. Anatomy, leaf, mesophyll, aquiferous hypodermis to chlorenchyma, abrupt transition: 0=absent; 1=present. 86. Anatomy, leaf, mesophyll, aquiferous hypodermis, cell shape: 0=rounded; 1=anticlinally elongated. 87. Anatomy, leaf, mesophyll, arm parenchyma, presence: 0=absent; 1=present. 88. Anatomy, leaf, mesophyll, arm parenchyma, arm length: 0=short arms, low lacunosity; 1=long arms, high lacunosity.89. Anatomy, leaf, vascular bundles, fiber cap, presence: 0=absent; 1=present. 90. Anatomy, leaf, mesophyll, parenchyma sheath, radiate disposition, presence: 0=absent; 1=present. 91. Anatomy, leaf, vascular bundle, small bundles, fiber projections: 0=wider than tall; 1=taller than wide. 92. Anatomy, leaf, mesophyll, palisade parenchyma, presence: 0=absent; 1=present. 93. Anatomy, leaf, mesophyll, extravascular fibers, presence: 0=absent; 1=present. 94. Anatomy, leaf, extravascular fibers, position: 0=both faces; 1=only adaxial face; 2=only abaxial face. 95. Anatomy, leaf, adaxial face, mesophyll, number of layers of extravascular fibers: 0=one layer; 1=more than one layer. 96. Anatomy, leaf, abaxial face, mesophyll, number of layers of extravascular fibers: 0=one layer; 1=more than one layer. 97. Androecium, stamen, pollen, pollen aperture type: 0=biporate; 1=sulcate; 2=polyporate; 3=inaperturate. 98. Androecium, stamen, pollen, pollen grain size: 0=40-50 μm; 1=bigger or equal to 60 μm. 99. Androecium, stamen, pollen, exine sculpture: 0=reticulate; 1=foveolate; 2=smooth. 100. Androecium, stamen, pollen, lumen shape of exine sculptures: 0=rounded; 1=polygonal; 2=heterobrocate; 3=perfurate. 101. Androecium, stamen, pollen, exine pores: 0=equal or less than 1/3 of pollen diameter; 1=wider than half of pollen diameter.
Rights and permissions
About this article
Cite this article
Santos-Silva, F., Venda, A.K.L., Hallbritter, H.M. et al. Nested in chaos: Insights on the relations of the ‘Nidularioid Complex’ and the evolutionary history of Neoregelia (Bromelioideae-Bromeliaceae). Brittonia 69, 133–147 (2017). https://doi.org/10.1007/s12228-017-9460-x
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12228-017-9460-x