Abstract
Eleocharis carniolica W.D.J. Koch (Cyperaceae) is an endangered wetland spike rush mainly threatened by habitat loss and fragmentation. Understanding the germination ecology of this species is essential to perform successful conservation and restoration actions. In this study, we investigated the effect of vernalization (i.e. cold stratification), gibberellic acid (GA3) and chemical scarification on seed germination of E. carniolica from wild populations in northern Italy. The results showed that vernalization (i.e. 8-weeks at 4 °C) significantly improved germination probability, speed, and uniformity compared to non-stratified seeds. Gibberellic acid treatment alone or in combination with vernalization did not show a significant improvement in germination. Chemical scarification using sodium hypochlorite increased germination probability, with 8 h of scarification showing the highest success rate. However, 24-h scarification had a negative impact on germination. Overall, vernalization was found to be the most effective method to enhance germination in E. carniolica. These findings provide valuable insights into the seed germination ecology of this endangered species, aiding in its exsitu conservation, propagation, and in-situ restoration efforts. Moreover, they have important implications on future germination dynamics of this endangered species, especially with predicted climate change scenarios.
Similar content being viewed by others
Introduction
Land use change is recognized as the primary cause of the degradation of natural ecosystems and biodiversity loss (Ellis et al. 2010; Foley et al. 2011). Among the various ecosystems, wetland areas have suffered a drastic area reduction during recent centuries, with an estimated global area loss of at least 50% since 1900. This contraction is usually linked with agricultural drainage (Davidson 2014) to convert wetlands to productive fields. The disruption of these unique habitats has drastic consequences on biodiversity, threatening many wetland species and highlighting the need for effective conservation and restoration actions (Semlitsch and Bodie 1998; Mosanghini et al. 2023). Therefore, the study of adequate conservation and restoration strategies for endangered wetland plants is crucial to maintain present biodiversity levels and avoid species extinction (Gibbs 2000). In this light, developing effective ex situ seed conservation and germination protocols for endangered species is of the utmost importance (Godefroid et al. 2011; Shen et al. 2015).
Seed germination is recognized as a pivotal stage in a plant's life cycle (Dekkers et al. 2013), beginning when a seed emerges from its dormant state and starts to develop into a seedling (Baskin and Baskin 1998; Khurana and Singh 2001; Fenner and Thompson 2005). The success of seed germination is essential for the perpetuation of plant populations, affecting the plant community assembly (Trotta et al. 2023) and, ultimately, the maintenance of biodiversity in natural ecosystems (Herranz et al. 2010).
Orthodox seeds (i.e. those capable of surviving drying and storage at low temperature) must overcome the dormancy stage which is crucial to enhance the germination rate of the species. Seed dormancy is considered as the incapacity of a viable seed to germinate when the conditions are favorable (Bewley 1997; Finch-Savage and Leubner-Metzger 2006). In the literature several methods are proposed to overcome seed dormancy. In particular, treatments such as cold stratification (i.e. vernalization) (Zhou et al. 2009; Vandelook et al. 2009), chemical or mechanical scarification (Patanè and Gresta 2006; Chisha-Kasumu et al. 2007; Olmez et al. 2007; Wang et al. 2007) and use of active molecules (e.g. gibberellic acid; GA3) (Tigabu and Oden 2001; Nadjafi et al. 2006) have been used to improve germination probability, germination speed, and uniformity. The endogenous growth regulating hormone GA3, for instance, has been proven to be crucial to stimulating seed germination (Debeaujon and Koornneef 2000; Graeber et al. 2012) by increasing the growth potential of the embryo and by inducing hydrolytic enzymes (Ogawa et al. 2003; Kucera et al. 2005; Finch-Savage and Leubner-Metzger 2006). Moreover, it is accepted that vernalization is able to break down dormancy by decreasing the level of abscisic acid while increasing the amount of gibberellic acid in the embryo (Feurtado et al. 2004; Graeber et al. 2012). Dormancy can also be manually released by removing constraints (i.e. tissues that surround the embryo) preventing germination (i.e. scarification) (Graeber et al. 2012).
Understanding the germination probability of plant species is crucial for identifying key limiting factors that may hinder natural regeneration or restoration efforts (Angevine and Chabot 1979; Narbona et al. 2013; Vuerich et al. 2022). By identifying such obstacles, conservationists can implement targeted interventions, such as habitat restoration, habitat connectivity improvements, or seed banking, to enhance germination success and promote the long-term survival of endangered plant species. Moreover, the speed of seed germination represents a pivotal determinant of a plant species' survival in its natural environment. In harsh ecosystems, where resources are limited and environmental conditions fluctuate, early germination can provide a significant advantage to a plant species (Gioria and Pyšek 2017; Gioria et al. 2018). Rapid germination enables seedlings to establish and access vital resources such as light, water and nutrients before other competing species (Benech-Arnold et al. 2000; Forbis 2010). This early establishment bolsters a plant's resilience against environmental stressors and enhances its ability to compete with other native and alien species and thrive in challenging conditions (Mosanghini et al. 2023), thereby increasing its overall chances of survival.
Eleocharis carniolica is one of the most endangered and rarest species of the European wetland flora, included in Habitat Directive Annex II (92/43/CEE). Previous studies have highlighted that E. carniolica produces dormant seeds (Puchalski et al. 2014; Niemczyk et al. 2023); thus it is crucial to conduct experiments aiming at optimizing the seed dormancy breaking procedures with appropriate treatments. Other species of the genus Eleocharis have been reported to exhibit irregular germination rates under natural conditions and difficulties in ex situ germination (Leeds et al. 2006; Baskin and Baskin 2014; Rosbakh et al. 2019). Since reproduction by seed is more desirable for successful maintenance of genetic diversity and optimal population reinforcement, the establishment of effective germination protocols is required. Therefore, urgent ex situ conservation actions have been undertaken for its preservation (specifically, the EU LIFE project (2021) LIFE20 NAT/IT/001468, LIFE SEEDFORCE; Magrini et al. 2022).
In this context we designed an in vitro germination experiment aimed at shedding new light on the critical factors that contribute to the speed and probability of seed germination of the endangered species E. carniolica. We treated seeds of this species with vernalization, gibberellic acid and chemical scarification treatments to induce seed germination and we hypothesized that all the treatments affect germination success (following Rosbakh et al. 2019). In particular, we expected vernalization and gibberellic treatment to positively interact to determine the germination success, as most wetland species show a nondeep physiological class of dormancy overcome by vernalization and/or GA3 (Baskin and Baskin 2014).
Materials and methods
Study species
Eleocharis carniolica is included in the EU Habitats Directive list (EEC, 1992, annex II) and is listed in the European Red List of Vascular Plants (Abeli et al. 2011). The species range is mainly centered in the central-east part of Europe, with the richest populations occurring in Hungary (Lansdown 2011). Other important populations have been recorded in Slovenia, Croatia, Romania (Anca et al. 2007; Lansdown 2011; Šegota and Alegro 2016), Bulgaria, Turkey, Slovakia (Lansdown 2011), Austria, Bosnia (Đug et al. 2013) and Montenegro (Vuksanović et al. 2019). In Italy the species has a scattered distribution across the northern part of the country, mainly across the Alpine chain and its southern foothills. Moreover, Italian populations appear to be isolated from other European populations because of the Alpine barrier (Gennai et al. 2013). Although at a global level the species has an IUCN conservation status of Least Concern (Lansdown 2011), in Italy it is considered Endangered (Gennai et al. 2013). Nowadays the species has a status of ‘U1 Unfavorable – Inadequate’ (EEA 2018; EUNIS 2018). Populations in the Lombardy and Friuli Venezia Giulia regions are at risk of extinction or size reduction, with some of the main threats being land use change and reforestation of abandoned wet and marshy meadows (Brusa and Ammiraglio 2021).
E. carniolica is a caespitose perennial plant (Fitter 1980; Verloove 2010), but it also behaves as an annual (Lastrucci and Becattini 2009). The optimum habitats of this species are wet meadows (Pignatti et al. 2017), lake or pond shores, temporary puddles, muddy habitats (Martini 1985; Lastrucci and Becattini 2007, 2008), and anthropogenic sites such as flooded fields or road verges and drainage waterways (Niemczyk et al. 2023). It is a pioneer species on disturbed bare soils and herbaceous open areas at low altitude (Barina et al. 2011). It prefers clayey, oligotrophic, acidic soils. It is an anemophilous species, and the dispersion of the seed is thought to be via hydrochory.
Fruit collection and storage
Achenes (treated as seeds) of E. carniolica were collected at the time of natural dispersal from two wild populations in northern Italy: the first one is occurring in the “Palude di Racchiuso” protected area (SAC: IT3320039; 46°10′0.48’’N 13°18′37.08’’E), in oligo- to meso-trophic standing waters (August 2022) and the second one from the Groane Regional Park (SAC IT2050002 Boschi delle Groane; 45°36′43.97"N 9°5′59.85"E)(August 2022). The two populations are considered to be among the most important in the Italian regions of Friuli Venezia Giulia and Lombardy, being estimated at thousands of ramets and also representing the eastern and western boundary of the species range in the Po Plain, respectively. The sites are donor sites for the LIFE project LIFE20 NAT/IT/001468.
The collected seeds were initially stored in a dry room in the laboratory for one month, then cleaned and stored dry at room temperature until the start of vernalization treatment (December 2022) and germination experiments (February 2023).
Experimental design
Two sets of experiments on seed from each population were performed in parallel both at the University of Udine and CFA Monte Barro facilities, respectively.
Firstly, a two-factorial combination of vernalization (i.e. cold stratification) and hormonal priming with gibberellic acid (GA3) as dormancy-breaking treatments was tested. For vernalization, seeds were subjected to 8-week cold/wet stratification in Falcon tubes at 4 °C (Baskin and Baskin 2014). For treatment with GA3, seeds were soaked in 1000 ppm GA3 (Sigma Aldrich) solution for 24 h. In particular, 4 sets of seeds were prepared: seeds placed in Petri dishes without a prior stratification period or GA3 treatment (hereafter CONTROL), seeds without a prior stratification with GA3 treatment (hereafter GA3), seeds wet vernalized without GA3 treatment (hereafter VERN) and seeds that were wet vernalized and then subsequently GA3 treated (hereafter VERN + GA3). For all treatments, seeds were firstly surface-sterilized with commercial bleach (NaClO, 4.5% w/v active chlorine) for 10 min and then rinsed several times with sterile milliQ water.
A further experiment was conducted to determine the effect of chemical scarification on seed dormancy of different durations (0, 4, 8, 12 or 24 h). Seeds were incubated with 1 ml solution of commercial bleach (NaClO, 4.5% w/v active chlorine), for the corresponding duration period at room temperature in dark conditions and then thoroughly rinsed with sterile milliQ water before germination testing.
For each treatment and population, sets of 20 seeds in six replicates were prepared (6 replicates × 20 seeds × 8 treatments × 2 populations with a total number of 1920 seeds).
Seed germination test
Seeds were placed on water agar (1%, w/v agar) in sterile 6 cm-diameter Petri dishes, closed with Parafilm to limit moisture loss and incubated at alternating temperatures and photoperiod (25 and 22 °C with a photoperiod of 14 h day light and 10 h dark) in a growth cabinet for 35 days. These conditions were chosen as the most suitable for germination of Eleocharis species of temperate mudflat and swamp communities (Rosbakh et al. 2020), consistently to what observed in natural populations of Po Valley (Italy). The dishes were monitored at one-day intervals and seeds with visible protruding coleoptile emergence (Walters 1950; Baskin and Baskin 2018) were considered as germinated. Germinated and non-germinated seeds were left in the Petri dishes for the entire incubation period and at the end the total number of non-germinated seeds was counted.
Germination parameters and statistical analysis
The germination records were analysed considering the different treatments in the two corresponding sets of experiments by the log-logistic model, based on time-to-event analysis (Onofri et al. 2010, 2022). This model provided inferences on germination events that did not occur at the specific time of evaluation, but during the interval between evaluations (Ritz et al. 2013). Three germination parameters were determined by the following equation:
where F(t) is the fraction of germinated seeds between time interval t, d is the parameter referring to the total germination (i.e. the higher asymptote of the function), b is the slope of the curve at the inflection point (i.e. usually described as seed uniformity), and e is the time required for 50% seed germination (usually known as T50), here used as a measure of germination rate.
All the statistical analyses were performed in the R environment (R Core Team 2022). The dataset preliminary was set for analyses using the function “mel_te” (i.e. reshaping time-to-event datasets) of the “drcte” R package (Onofri et al. 2022), derived from the “drc” package (Ritz et al. 2015). The model was executed using the “drmte” function (i.e. fitting time-to-event models for seed science). The difference between the 3 germinating parameters (i.e. b, d and e) between the two different treatments (i.e. gibberellic acid and vernalization) were tested with the function “compCDF” (i.e. compare time-to-event curves, comparing the difference in each model). The difference was considered statistically significant when the p-value was < 0.05.
Results
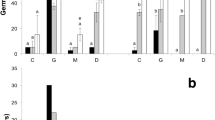
The results are presented in two sections following the different sets of experiments we performed. Firstly, we analysed the germination performance of Eleocharis carniolica with vernalization, gibberellic acid and the interaction between these treatments; secondly, we studied the germination performance with increasing amounts of time of scarification. The germination curves are presented respectively in Figs. 1 and 2. The full report of the germination percentage over time is reported in Table S1 and S2.
Seed germination curve for vernalization and gibberellic acid treatments: vernalized seeds (blue, referred as VERN), non-vernalized seeds (red), the filled points and lines represent the gibberellic acid treatment (referred to as GA3), and the empty points and dashed lines represent the seeds without gibberellic acid. The treatment described as VERN + GA3 refers to the combined effects of vernalization and gibberellic acid. The circles represent the observed germination proportion; the lines (cumulative probability of germination) show the results of fitting equation 1 to the data
Seed germination curves for the chemical scarification treatment. The number of hours of exposure to chemical scarificant is represented by the increasing darkness of the line color, corresponding to an increase in treatment time. The lines (cumulative probability of germination) show the results of fitting equation 1 to the data
Effects of vernalization and GA3 on seed germination parameters of Eleocharis carniolica
The vernalization significantly enhanced the germination performance of E. carniolica (Table 1). In particular, vernalization increased the germination probability, showing a significant higher value than in other treatments (control and vernalization; Table 1). Although the highest germination probability was achieved when both seed vernalization and gibberellic acid were used (78% success; Table 2), the difference of the combined treatment with vernalization was not statistically significant. Interestingly, the control exhibited a higher gemination probability compared with the seed treated only with GA3 (35% instead of 27%).
Moreover, vernalization promoted a significant increase of germination speed, with a sensible lower value of the e parameter (i.e. T50, 4.71 days) compared with the GA3 treatment and the control.
Finally, vernalization increased the uniformity of seed germination of E. carniolica. The other treatments (i.e. vernalization with GA3, control and GA3) showed no significant differences.
Effects of increasing scarification time on seed germination parameters of Eleocharis carniolica
These tests highlighted that 8 h of scarification appears to be the treatment with the highest germination probability (i.e. 55%), with differences as compared to all the other treatments except for 12 h scarification (p-value = 0.11; Table 3). The less effective treatment was the 24 h scarification, with 23% germination success (Table 4). The 4 h treatment showed similar behaviour, in terms of germination probability, compared with the control.
Interestingly, the analyses did not reveal any significant differences between the various treatments and the germination speed, except for the 24 h scarification. This result appears to be the slowest found, with a T50 (i.e. e parameter) of more than 10 days.
Moreover, scarification appears to be a key factor in increasing the uniformity of seed germination. Indeed, the various time treatments appear not to be statistically different compared to one another. The difference in uniformity was found only when compared with the untreated seeds (i.e. control), resulting in a decrease of uniformity.
Discussion
Our results showed that E. carniolica in northern Italian populations produces dormant seeds and that germination is strictly related to seed vernalization (i.e. cold stratification). We also demonstrated that seed germination was not increased by further treatment by 1000 ppm GA3 nor by its interaction with vernalization. Our results thus suggest that seed needs to undergo cold temperature to interrupt dormancy, rising some important considerations concerning conservation and germination protocols for E. carniolica.
In other species of the genus Eleocharis and in other Cyperaceae, irregular germination rates in the wild and difficult ex situ germination in the laboratory are also reported (Grime et al. 1981; Leeds et al. 2006; Baskin and Baskin 2014; Rosbakh et al. 2019). In particular, Baskin and Baskin (2014) classified most species of Eleocharis as physiologically dormant. Seed dormancy is also confirmed for E. carniolica from Polish populations (Puchalski et al. 2014; Niemczyk et al. 2023) even though a specific investigation on the class of dormancy was not achieved. It is suggested that this behaviour could be related to physiological dormancy (embryo-dormancy) as an adaptation to adverse prolonged periods of low temperatures or also to physical (pericarp-induced) dormancy due to a particularly hard coat, resistant to either acidic soil pH, frost damage, or gastric juices of frugivorous birds (Schütz 2000; Webb et al. 2009).
In the Seed Information Database, optimal germination conditions are available for a small number of Eleocharis species among the fifty two present (Society for Ecological Restoration, International Network for Seed Based Restoration and Royal Botanic Gardens Kew 2023). For E. palustris (L.) Roem. & Schult., a related native species belonging to the same Eleocharis section and subgenus (González-Elizondo and Peterson 1997), a 50% germination percentage and a very long germination timing (42 days) were reported for non-treated seeds, while an 18-week vernalization preferably after bleach pretreatment (2–6% for 6–48 h duration) is recommended for breaking dormancy (Rosbakh et al. 2019).
According to our hypothesis, in the present study, timing, uniformity, and extent of germination in E. carniolica seed from two populations was effectively improved by vernalization, as the probability of germination was doubled in VERN seeds, independently from GA3 application, compared to non-stratified seeds. Consistently, VERN seeds were also germinated more rapidly, homogenously and consistently, confirming that moist vernalization not only released seed dormancy, but also increased the final seed vigour for seed from different populations of E. carniolica. These qualitative aspects, explaining the dynamics of the germination process, are fundamental for the optimal ecological restoration of such endangered species (Talská et al. 2020). Concerning the positive effect of vernalization, it is proposed that seeds of various wetland species can be successfully stored at low temperatures (0–8 °C) in water or cold wet stratification for 6–9 weeks. This process simulates the situation in nature when the mature seeds are shed from plant on wet soils subjected to waterlogging and cold winter temperatures (Baskin and Baskin 2014), ensuring a prompt spring germination. Similar to several Carex species of temperate wetlands, Eleocharis could also take advantage of exploring spatially and temporally occasional gaps in vegetation that occur only in late spring-early summer. Colonization of these vegetation gaps, in fact, would not be prevented by other competitive species that in contrast have more specific high temperature requirements in order to germinate (Schütz 2000). The lack of vegetation cover would also promote higher light conditions, which are commonly favourable in promoting seed germination in many wetland and disturbed areas species.
A clear positive effect of hormone treatment with GA3 in breaking dormancy, alone or in combination with vernalization, was not found, since GA3 non-vernalized seeds were significantly more rapid in germinating when pre-treated with GA3, but the final extent of germination was lower. Instead, in VERN + GA3 combination, hormone pre-treatment significantly increased only their seed uniformity. This is different from what happens in seeds with nondeep physiological dormancy, in which dormancy can be overcome by chemicals, like GA3, alone or also in combination with cold stratification (Baskin and Baskin 2014). These results would suggest a complex scenario, where physiological embryonic dormancy could be a component in dormancy behaviour in the populations of northern Italy (Schütz 2000) but not strictly related to an insufficient level of GA3, or an intermediate physiological type of dormancy (Baskin and Baskin 2014). Seeds with this type of dormancy have a fully developed embryo, but the seed coat or pericarp may slow the hydration rate or diffusion of oxygen and the effect of GA3 on germination is variable. In contrast to our results, Niemczyk et al. (2023) recommended a brief (one week) warm stratification in darkness with the application of 300 ppm GA3 as the most effective treatment for overcoming seed dormancy for Polish populations of E. carniolica and they suggested that light was also pivotal for seeds to initiate germination. However, our contrasting results demonstrating a requirement for cold temperature to overcome dormancy in E. carniolica species could be linked to the genetic isolation of the Italian populations from the central and east European ones due to the Alpine barrier (Gennai et al. 2013). Inter-population variation in seed dormancy of plant species has already been identified (Cochrane et al. 2015), being especially beneficial for the survival of populations in changing environments and highly variable habitats, such as temporary wetlands with sometimes unpredictable alternation in flooding and dry periods (Carta et al. 2013). Thus, it could be speculated that the variation in dormancy-breaking requirements between Polish and Po Valley (Italy) populations represents specific adaptive responses to similar habitats but within an extended geographic range with continental to temperate climatic regimes. In fact, dormancy breaking is one of the critical early life stages, along with germination and seedling establishment, which drive population response to environmental pressure and climate change, and plant species distributed over temporally heterogeneous environments (e.g. those with high daily or periodic fluctuations in flooding or temperature) are predicted to have more plasticity in these traits (Finch et al. 2019). However, it cannot be ruled out that different seed storage conditions (15 °C vs. room temperature, i.e., 25 °C) applied prior to stratification experiments may have altered their responses in terms of the required temperature for seed dormancy break (Baskin and Baskin 2022).
Lastly, it has been suggested that chemical scarification with sodium hypochlorite could also break seed dormancy alone or in combination with vernalization by promoting water uptake after removal/dissolution or oxidation of the harder integument or inhibitory components present in the pericarp of several Eleocharis or other Cyperaceae species (Yeo and Thurston 1983; Yeo 1986; Leeds et al. 2006; Webb et al. 2009; Rosbakh et al. 2019, 2020). Similarly, we found that chemical scarification significantly increased the probability of germination in E. carniolica compared to the control for all duration periods apart from 24 h, though never reaching the highest value observed with vernalization (55 vs. 73%) and not affecting germination speed and uniformity. The mechanism of bleaching in promoting dormancy release is unclear and some possible explanations are proposed. It could be associated to degradation of the seed coat required to start the germination process, in accordance to microscopy observations by Leeds et al. (2006) and also in E. carniolica in the present study, where bleach-treated seed coats showed a gel-like appearance. Another explanation could be linked to an oxidising effect, given that sodium hypochlorite in solution also releases oxygen gas as a byproduct (Bewley and Black 1982; Yeo 1986). It is suggested that bleach scarification could enhance germination by degrading wax-like substances present in the pericarp in E. coloradoensis, while mechanical methods of scarification were only partially successful (Yeo and Thurston 1983). In addition, bleach improves lignin degradation of seed coat cells by oxidation, thus increasing water imbibition of the seed (Pierce et al. 2019). Otherwise, hypochlorite stimulates oxidative signalling, like reactive oxygen species, which commonly plays a positive role in dormancy release to some extent (Farooq et al. 2021). Rosbakh et al. (2020) reported that several Eleocharis species characteristic of muddy swamps and ponds require aerobic conditions for germination, in addition to fluctuating light, water and temperature. Therefore, chemical scarification could simulate what happens in nature, when seeds buried in muddy soils are occasionally exposed to air and light after the passage of hoofed animals or agricultural machineries or mimic the effect of passage of seeds through the digestive gut of wildfowl (Leeds et al. 2006). However, careful selection of the optimal exposure and proper concentration of bleach is needed, in order to avoid the opposite effect resulting in a reduction in germination performance, as observed in 24 h-bleach treated seeds, due to full pericarp loss or damage of the embryo (Marty and Kettenring 2017).
In conclusion, we confirmed that E. carniolica seeds from populations of northern Italy can be stimulated by dormancy-releasing pre-treatments of vernalization and, moderately, from chemical scarification to some extent, similar to several other wetland Cyperaceae and Eleocharis species that exhibit scarce ex situ germination.
These results provide the basis for understanding the seed germination ecology of this endangered species and for developing optimal procedures for its conservation, propagation and cultivation in ex situ conservation strategies and in-situ restoration and reinforcement projects.
References
Abeli T, Acevedo Rodríguez A, Aguiar C (2011) European red list of vascular plants. Publications Office of the European Union
Anca S, Oprea A, and Sârbu I (2007) PLANTS FROM THE HABITAT DIRECTIVE–ANNEX IIb, PRESENTS IN ROMANIA. J Plant Dev
Angevine MW, Chabot BF (1979) Seed Germination Syndromes in Higher Plants. In: Solbrig OT, Jain S, Johnson GB, Raven PH (eds) Topics in Plant Population Biology. Palgrave, London. https://doi.org/10.1007/978-1-349-04627-0_9
Barina Z, Pifkó D, Mesterházy A (2011) Contributions to the flora of Albania, 3. Willdenowia 41(2):329–339. https://doi.org/10.3372/wi.41.41214
Baskin CC, Baskin JM (1998) Seeds: ecology, biogeography, and evolution of dormancy and germination. Elsevier
Baskin CC, Baskin JM (2014) Seeds: ecology, biogeography, and evolution of dormancy and germination, 2nd edn. Elsevier, London
Baskin CC, Baskin JM (2018) Resolving the puzzle of Martin’s broad embryo: a solution based on morphology, taxonomy and phylogeny. Perspect Plant Ecol Evol Syst 34:61–67. https://doi.org/10.1016/j.ppees.2018.08.001
Baskin CC, Baskin JM (2022) Mimicking the natural thermal environments experienced by seeds to break physiological dormancy to enhance seed testing and seedling production. Seed Sci Technol 50:21–29. https://doi.org/10.15258/sst.2022.50.1.s.02
Benech-Arnold RL, Sánchez RA, Forcella F, Kruk BC, Ghersa CM (2000) Environmental control of dormancy in weed seed banks in soil. Field Crop Res 67(2):105–122
Bewley J (1997) Seed germination and dormancy. Plant Cell 9(7):1055–1066
Bewley JD, Black M (1982) Physiology and biochemistry of seeds in relation to germination. Springer, Berlin, Heidelberg
Brusa G, Ammiraglio S (2021) Stato delle conoscenze sulla distribuzione delle specie vegetali degli Allegati della Direttiva Habitat (92/43/CEE) in Lombardia: Eleocharis carniolica. 2° aggiornamento. In: Parco Monte Barro-Centro Flora Autoctona, Società Botanica Italiana - Sez. Lombarda, Osservatorio Regionale per la Biodiversità di Regione Lombardia. chrome-extension://efaidnbmnnnibpcajpcglclefindmkaj/http://www.biodiversita.lombardia.it/images/FLORA/pdf/Eleocharis-carniolica.pdf. Accessed 20 Jul 2023
Carta A, Bedini G, Müller JV, Probert RJ (2013) Comparative seed dormancy and germination of eight annual species of ephemeral wetland vegetation in a Mediterranean climate. Plant Ecol 214(2):339–349. https://doi.org/10.1007/s11258-013-0174-1
Chisha-Kasumu E, Woodward S, Price A (2007) Comparison of the effects of mechanical scarification and gibberellic acid treatments on seed germination in Pterocarpus angolensis. South Hemisphere For J 69(1):63–70. https://doi.org/10.2989/SHFJ.2007.69.1.9.171
Cochrane A, Yates CJ, Hoyle GL, Nicotra AB (2015) Will among-population variation in seed traits improve the chance of species persistence under climate change? Glob Ecol Biogeogr 24(1):12–24. https://doi.org/10.1111/geb.12234
Davidson NC (2014) How much wetland has the world lost? Long-term and recent trends in global wetland area. Mar Freshw Res 65(10):934–941
Debeaujon I, Koornneef M (2000) Gibberellin requirement for Arabidopsis seed germination is determined both by testa characteristics and embryonic abscisic acid. Plant Physiol 122(2):415–424
Dekkers BJW, Pearce S, van Bolderen-Veldkamp RP, Marshall A, Widera P, Gilbert J, Drost H-G, Bassel GW, Müller K, King JR, Wood ATA, Grosse I, Quint M, Krasnogor N, Leubner-Metzger G, Holdsworth MJ, Bentsink L (2013) Transcriptional dynamics of two seed compartments with opposing roles in Arabidopsis seed germination. Plant Physiol 163(1):205–215. https://doi.org/10.1104/pp.113.223511
Directive H (1992) Council Directive 92/43/EEC of 21 May 1992 on the conservation of natural habitats and of wild fauna and flora. Off J Eur Union 206(7):50
Đug S, Muratović E, Drešković N, Boškailo A, Dudević S (2013) Crvena lista flore Federacije Bosne i Hercegovine. EU “Greenway”, Sarajevo
EEA (2018) Species assessments at EU biogeographical level - European Environment Agency. https://nature-art17.eionet.europa.eu/article17/species/summary/?period=5&subject=Eleocharis%20carniolica. Accessed 18 Aug 2023
Ellis EC, Klein Goldewijk K, Siebert S, Lightman D, Ramankutty N (2010) Anthropogenic transformation of the biomes, 1700 to 2000. Glob Ecol Biogeogr 19(5):589–606
EU LIFE project (2021) LIFE20 NAT/IT/001468 LIFE SEEDFORCE. In: LIFE Public Database. https://webgate.ec.europa.eu/life/publicWebsite/project/details/5736. Accessed 20 Jul 2023
EUNIS (2018) Eleocharis carniolica - W.D.J.Koch - European Nature Information System. https://eunis.eea.europa.eu/species/187643. Accessed 18 Aug 2023
Farooq MA, Zhang X, Zafar MM, Ma W, & Zhao J (2021). Roles of reactive oxygen species and mitochondria in seed germination. Front Plant Sci 12:781734
Fenner M, Thompson K (2005) The ecology of seeds. Cambridge University Press
Feurtado JA, Ambrose SJ, Cutler AJ, Ross AR, Abrams SR, Kermode AR (2004) Dormancy termination of western white pine (Pinus monticola Dougl. Ex D. Don) seeds is associated with changes in abscisic acid metabolism. Planta 218:630–639
Finch J, Walck JL, Hidayati SN, Kramer AT, Lason V, Havens K (2019) Germination niche breadth varies inconsistently among three Asclepias congeners along a latitudinal gradient. Plant Biol 21(3):425–438. https://doi.org/10.1111/plb.12843
Finch-Savage WE, Leubner-Metzger G (2006) Seed dormancy and the control of germination. New Phytol 171(3):501–523. https://doi.org/10.1111/j.1469-8137.2006.01787.x
Fitter AH (1980) Flora Europaea, Vol. 5, edited by TG Tutin et al. Cambridge UP,£ 37.50. Oryx 15(4):413–413
Foley JA, Ramankutty N, Brauman KA, Cassidy ES, Gerber JS, Johnston M, Mueller ND, O’Connell C, Ray DK, West PC (2011) Solutions for a cultivated planet. Nature 478(7369):337–342
Forbis TA (2010) Germination phenology of some Great Basin native annual forb species. Plant Species Biol 25(3):221–230. https://doi.org/10.1111/j.1442-1984.2010.00289.x
Gennai M, Lastrucci L, Selvaggi A, Castello M (2013) Schede per una Lista Rossa della Flora vascolare e crittogamica Italiana: Eleocharis carniolica Koch. Informatore Botanico Italiano 45(1):146–149
Gibbs JP (2000) Wetland loss and biodiversity conservation. Conserv Biol 14(1):314–317
Gioria M, Pyšek P (2017) Early bird catches the worm: germination as a critical step in plant invasion. Biol Invasions 19(4):1055–1080. https://doi.org/10.1007/s10530-016-1349-1
Gioria M, Pyšek P, Osborne BA (2018) Timing is everything: does early and late germination favor invasions by herbaceous alien plants? J Plant Ecol 11(1):4–16. https://doi.org/10.1093/jpe/rtw105
Godefroid S, Piazza C, Rossi G, Buord S, Stevens A-D, Aguraiuja R, Cowell C, Weekley CW, Vogg G, Iriondo JM, Johnson I, Dixon B, Gordon D, Magnanon S, Valentin B, Bjureke K, Koopman R, Vicens M, Virevaire M, Vanderborght T (2011) How successful are plant species reintroductions? Biol Cons 144(2):672–682. https://doi.org/10.1016/j.biocon.2010.10.003
González-Elizondo MS, Peterson PM (1997) A classification of and key to the Supraspecific Taxa in Eleocharis (Cyperaceae). Taxon 46(3):433–449. https://doi.org/10.2307/1224386
Graeber K, Nakabayashi K, Miatton E, Leubner-Metzger G, Soppe WJJ (2012) Molecular mechanisms of seed dormancy. Plant Cell Environ 35(10):1769–1786. https://doi.org/10.1111/j.1365-3040.2012.02542.x
Grime JP, Mason G, Curtis AV, Rodman J, Band SR (1981) A Comparative study of germination characteristics in a local flora. J Ecol 69(3):1017–1059. https://doi.org/10.2307/2259651
Herranz JM, Ferrandis P, Martínez-Duro E (2010) Seed germination ecology of the threatened endemic Iberian Delphinium fissum subsp. sordidum (Ranunculaceae). Plant Ecol 211(1):89–106. https://doi.org/10.1007/s11258-010-9775-0
Khurana E, Singh JS (2001) Ecology of seed and seedling growth for conservation and restoration of tropical dry forest : a review. Environ Conserv 28(1):39–52. https://doi.org/10.1017/S0376892901000042
Kucera B, Cohn MA, Leubner-Metzger G (2005) Plant hormone interactions during seed dormancy release and germination. Seed Sci Res 15(4):281–307
Lansdown RV (2011) Eleocharis carniolica. The IUCN Red List of Threatened Species 2011:e.T161832A5501732. https://doi.org/10.2305/IUCN.UK.2011-1.RLTS.T161832A5501732.en. Accessed 10 Aug 2023
Lastrucci L, Becattini R (2009) La vegetazione delle aree umide presso Bosco ai Frati (Firenze, Toscana). Atti Soc Tosc Sci Nat mem Ser B 115 (2008):57–67
Lastrucci L, Becattini R (2007) Eleocharis carniolica Koch (Cyperaceae) new to Tuscany (Central Italy) and distribution of the allied species. Webbia 62(1):11–25. https://doi.org/10.1080/00837792.2007.10670814
Lastrucci L, Becattini R (2008) La vegetazione delle aree umide presso Bosco ai Frati (Firenze (Toscana). Atti Soc tosc Sci nat, Mem 115(Serie B):56–57
Leeds JA, Newman S, Smith SM (2006) Factors affecting seed germination of Eleocharis cellulosa and Rhyncospora tracyi from the northern Everglades. Wetlands 26(2):368–375. https://doi.org/10.1672/0277-5212(2006)26[368:FASGOE]2.0.CO;2
Magrini S, Bonomi C, Bacchetta G, Bedini G, Borzatti A, Boscutti F, Carasso V, Carta A, Casavecchia S, Casolo V, Ceriani R, Cristaudo A, Di Cecco V, Di Martino L, Digangi I, Fabrini G, Guglielmo F, Mariotti M, Negri V, Porceddu M, Villani M, Zappa E, Salmeri C (2022) The RIBES strategy for ex situ conservation: conventional and modern techniques for seed conservation. Flora Mediterranea 32:395–401. https://doi.org/10.7320/FlMedit32.395
Martini F (1985) Appunti sulla flora delle Alpi Friulane e del loro avanterra. Gortania- Atti Museo Friulano di Storia Naturale 6:147–174
Marty JE, Kettenring KM (2017) Seed dormancy break and germination for restoration of three globally important wetland bulrushes. Ecol Restor 35(2):138–147. https://doi.org/10.3368/er.35.2.138
Mosanghini D, Oriolo G, Boscutti F (2023) Different ways to success: Plant community trajectories over time and a soil moisture gradient in restored wetlands. J Appl Ecol 60(1):29–40. https://doi.org/10.1111/1365-2664.14308
Nadjafi F, Bannayan M, Tabrizi L, Rastgoo M (2006) Seed germination and dormancy breaking techniques for Ferula gummosa and Teucrium polium. J Arid Environ 64(3):542–547. https://doi.org/10.1016/j.jaridenv.2005.06.009
Narbona E, Delgado A, Encina F, Miguez M, Buide ML (2013) Seed germination and seedling establishment of the rare Carex helodes Link depend on the proximity to water. Aquat Bot 110:55–60
Niemczyk M, Rucińska A, Puchalski J, Kapler A, Nowak A, Jaźwa M (2023) Is the protection of habitat directive Eleocharis carniolica in its northern limits really needed? – A life strategy based investigation. Aquat Bot 188:103676. https://doi.org/10.1016/j.aquabot.2023.103676
Ogawa M, Hanada A, Yamauchi Y, Kuwahara A, Kamiya Y, Yamaguchi S (2003) Gibberellin biosynthesis and response during Arabidopsis seed germination. Plant Cell 15(7):1591–1604
Olmez Z, Gokturk A, Temel F (2007) Effects of cold stratification, sulphuric acid, submersion in hot and tap water pretreatments on germination of bladder-senna (Colutea armena Boiss. & Huet.) seeds. Seed Sci Technol 35(2):266–271. https://doi.org/10.15258/sst.2007.35.2.02
Onofri A, Gresta F, Tei F (2010) A new method for the analysis of germination and emergence data of weed species. Weed Res 50(3):187–198. https://doi.org/10.1111/j.1365-3180.2010.00776.x
Onofri A, Mesgaran MB, Ritz C (2022) A unified framework for the analysis of germination, emergence, and other time-to-event data in weed science. Weed Sci 70(3):259–271. https://doi.org/10.1017/wsc.2022.8
Patanè C, Gresta F (2006) Germination of Astragalus hamosus and Medicago orbicularis as affected by seed-coat dormancy breaking techniques. J Arid Environ 67(1):165–173. https://doi.org/10.1016/j.jaridenv.2006.02.001
Pierce S, Spada A, Caporali E, Ceriani RM, Buffa G (2019) Enzymatic scarification of Anacamptis morio (Orchidaceae) seed facilitates lignin degradation, water uptake and germination. Plant Biol 21(3):409–414. https://doi.org/10.1111/plb.12694
Pignatti S, Guarino R, La Rosa M (2017). Flora d'Italia. ISBN 978-88-506-5242-6
Puchalski J, Niemczyk M, Walerowski P, Podyma W, Kapler A (2014) Seed banking of Polish endangered plants – the FlorNatur Project. Biodivers Res Conserv 34(1):65–72. https://doi.org/10.2478/biorc-2014-0005
R Core Team R (2022) R: A language and environment for statistical computing
Ritz C, Pipper CB, Streibig JC (2013) Analysis of germination data from agricultural experiments. Eur J Agron 45:1–6. https://doi.org/10.1016/j.eja.2012.10.003
Ritz C, Baty F, Streibig JC, Gerhard D (2015) Dose-response analysis using R. PLOS ONE 10(12):e0146021. https://doi.org/10.1371/journal.pone.0146021
Rosbakh S, Hülsmann L, Weinberger I, Bleicher M, Poschlod P (2019) Bleaching and cold stratification can break dormancy and improve seed germination in Cyperaceae. Aquat Bot 158:103128. https://doi.org/10.1016/j.aquabot.2019.103128
Rosbakh S, Phartyal SS, Poschlod P (2020) Seed germination traits shape community assembly along a hydroperiod gradient. Ann Bot 125(1):67–78. https://doi.org/10.1093/aob/mcz139
Schütz W (2000) Ecology of seed dormancy and germination in sedges (Carex). Perspect Plant Ecol Evol Syst 3(1):67–89. https://doi.org/10.1078/1433-8319-00005
Šegota V, Alegro A (2016) New finding of the red listed Eleocharis carniolica Koch in Croatia. Glasnik Hrvatskog Botaničkog Društva 4(1):30–31
Semlitsch RD, Bodie JR (1998) Are small, isolated wetlands expendable? Conserv Biol 12(5):1129–1133. https://doi.org/10.1046/j.1523-1739.1998.98166.x
Shen S-K, Wu F-Q, Yang G-S, Wang Y-H, Sun W-B (2015) Seed germination and seedling emergence in the extremely endangered species Rhododendron protistum var. giganteum—the world’s largest Rhododendron. Flora - Morphol Distrib Funct Ecol Plants 216:65–70. https://doi.org/10.1016/j.flora.2015.08.006
Society for Ecological Restoration, International Network for Seed Based Restoration and Royal Botanic Gardens Kew (2023) Seed Information Database (SID). In: Society for ecological Restoration. https://ser-sid.org/. Accessed 20 Jul 2023
Talská R, Machalová J, Smýkal P, Hron K (2020) A comparison of seed germination coefficients using functional regression. Appl Plant Sci 8(8):e11366. https://doi.org/10.1002/aps3.11366
Tigabu M, Oden PC (2001) Effect of scarification, gibberellic acid and temperature on seed germination of two multipurpose Albizia species from Ethiopia. Seed Sci Technol 29(1):11–20
Trotta G, Vuerich M, Petrussa E, Hay FR, Assolari S, Boscutti F (2023) Germination performance of alien and native species could shape community assembly of temperate grasslands under different temperature scenarios. Plant Ecol. https://doi.org/10.1007/s11258-023-01365-7
Vandelook F, Bolle N, Assche JAV (2009) Morphological and physiological dormancy in seeds of Aegopodium podagraria (Apiaceae) broken successively during cold stratification. Seed Sci Res 19(2):115–123. https://doi.org/10.1017/S0960258509301075
Verloove F (2010) Studies in Italian Cyperaceae 1. Eleocharis pellucida, new to Europe, naturalised in Piemonte (Italy). Webbia 65(1):133–140. https://doi.org/10.1080/00837792.2010.10670868
Vuerich M, Pasquini S, Casolo V, Boscutti F, Petrussa E (2022) Seed germination of the endemic Armeria helodes (Plumbaginaceae) in Italy. Flora Mediterranea 32:227–232. https://doi.org/10.7320/FLMEDIT32.227
Vuksanović S, Bubanja N, Berg C (2019) WDJ Koch, New Species in Flora of Montenegro. Hacquetia 18(1):129–135
Walters SM (1950) On the vegetative morphology of Eleocharis R. Br New Phytologist 49(1):1–7
Wang YR, Hanson J, Mariam YW (2007) Effect of sulfuric acid pretreatment on breaking hard seed dormancy in diverse accessions of five wild Vigna species. Seed Sci Technol 35(3):550–559. https://doi.org/10.15258/sst.2007.35.3.03
Webb J, Miao S, Zhang X-H (2009) Factors and mechanisms influencing seed germination in a wetland plant sawgrass. Plant Growth Regul 57(3):243–250. https://doi.org/10.1007/s10725-008-9341-0
Yeo RR (1986) Dormancy in seed of dwarf spikerush eleocharis coloradoensis. J Aquat Plant Manag 24:11–16
Yeo RR, Thurston JR (1983) Dormancy in seed of dwarf spikerush eleocharis coloradoensis. J Aquat Plant Manag 21(2):92–95
Zhou Z-Q, Bao W-K, Wu N (2009) Dormancy and germination in Rosa multibracteata Hemsl. & E. H. Wilson. Sci Hortic 119(4):434–441. https://doi.org/10.1016/j.scienta.2008.08.017
Funding
Open access funding provided by Università degli Studi di Trieste within the CRUI-CARE Agreement. The results presented in this paper were obtained with the financial support of the Friuli Venezia Giulia Region and Banca del Germoplasma Autoctono Vegetale del Friuli Venezia Giulia, and EU Life Project SEEDFORCE LIFE20 NAT/IT/001468. The study was also financially supported by Parco Monte Barro and Project LIFE IP GESTIRE 2020 via ERSAF Lombardia - Ente Regionale per i Servizi all’Agricoltura e alle Foreste. Thanks are also due to Michela Tomasella from Biodiversity Service of Region Friuli Venezia Giulia for logistic support and to Simon Pierce for critically reading of the paper and for support in in situ germplasm collection.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Trotta, G., Ceriani, R.M., Casolo, V. et al. Vernalization affects the germination performance of the wetland endangered species Eleocharis carniolica. Biologia 79, 729–738 (2024). https://doi.org/10.1007/s11756-024-01605-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11756-024-01605-9