Abstract
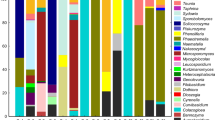
We studied the phylloplane-associated yeast communities of bromeliads at four sites in the Brazilian Northern Atlantic Forest through a culture-dependent approach and evaluated their potential as enzyme producers. A total of 213 isolates were identified by sequencing of D1/D2 region of the LSU rDNA gene or ITS region. Sequence analyses revealed that 182 isolates (85%) belong to the phylum Basidiomycota and 31 isolates (15%) to the phylum Ascomycota. The yeasts were identified as 86 species associated to 41 genera, reporting the highest phylloplane yeast richness in the literature to the present date. Besides, 59 strains were distributed in 32 possible undescribed species (38% of the total species in the study). Only two species occurred in all sampled sites: Carlosrosaea sp. nov. 3 and Papilliotrema flavescens, evidencing the heterogeneous character of yeast communities at these environments. At least one extracellular enzyme was detected in 173 isolates (85.6% of total yeast isolates), and one isolate of Aureobasidium thailandense was able to produce all five evaluated enzymes (amylase, cellulase, esterase, pectinase, and protease). The yeast community associated with bromeliads from the Atlantic Forest demonstrated a high heterogeneity and richness not yet found in previous studies on bromeliads, with a significant number of potential new species. Our results highlight bromeliads as a hotspot for yeast biodiversity studies.

Graphical abstract



Similar content being viewed by others
Data availability
The DNA sequences generated during and/or analyzed during the current study are available in the GenBank repository.
References
Altschul SF, Madden TL, Schäffer AA, Zhang J, Zhang Z, Miller W et al (1997) Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res 25(17):3389–3402
Andrews JH, Harris RF (2000) The ecology and biogeography of microorganisms on plant surfaces. Annu Rev Phytopathol 38(1):145–180. https://doi.org/10.1146/annurev.phyto.38.1.145
Araújo FV, Rosa CA, Freitas LFD, Lachance MA, Vaughan-Martini A, Mendonça-Hagler LC et al (2012) Kazachstania bromeliacearum sp. nov., a yeast species from water tanks of bromeliads. Int J Syst Evol Microbiol 62(Pt 4):1002–1006
Atlas RM, Parks LC (1993) Handbook of microbiological media. CRC Press, London
Azeredo LAI, Gomes EAT, Mendonça-Hagler LCS, Hagler AN (1998) Yeast communities associated with sugar-cane (Saccharum officinarum Linneau) near Campos, Rio de Janeiro, Brazil. Int Microbiol 1:205–208
Basílio GA, Elias D, Barbosa F, Furtado SG, Silva FR, Neto LM (2015) Community ecology of epiphytic Bromeliaceae in a remnant of Atlantic Forest in Zona da Mata, Minas Gerais State. Hoehnea 42(1):21–31
Bilinski CA, Stewart GG (1990) Yeasts proteases and brewing. In: Verachtert H, de Mot R (eds) Yeast biotechnology and Biocatalysised. Marcel Dekker, New York, pp 147–162
Bonifaz A, Badali H, de Hoog GS, Cruz M, Araiza J, Cruz MA, Fierro L, Ponce RM (2008) Tinea nigra by Hortaea werneckii, a report of 22 cases from México. Stud Mycol 61:77–82
Brandão LR, Vaz ABM, Espírito Santo LC, Pimenta RS, Morais PB, Libkind D, Rosa LH, Rosa CA (2017) Diversity and biogeographical patterns of yeast communities in Antarctic, Patagonian and tropical lakes. Fungal Ecol 28:33–43
Buzzini P, Martini A (2002) Extracellular enzymatic activity profiles in yeast and yeast-like strains isolated from tropical environments. J Appl Microbiol 93(6):1020–1025
Campanili M and Schaffer WB (2010) Mata Atlântica: manual de adequação ambiental. Brasília: MMA/SBF, 96 p, Série Biodiversidade, 35
Chen J, Xing XK, Zhang LC, Xing YM, Guo SX (2012) Identification of Hortaea werneckii isolated from mangrove plant Aegiceras comiculatum based on morphology and rDNA sequences. Mycopat 174(5–6):457–466
Crous PW, Wingfield MJ, Burgess TI, Hardy GESJ et al (2018) Fungal planet description sheets: 716–784. Persoonia 40:239–392
Crous PW, Carnegie AJ, Wingfield MJ, Sharma R (2019) Fungal planet description sheets: 868–950. Persoonia 42:291–473
Dizy M, Bisson LF (2000) Proteolytic activity of yeast strains during grape juice fermentation. Am J Enol Vitic 51:155–167
Felix CR, Casanova Navarro HM, Paulino GVB, Broetto L, Landell MF (2017) Carlosrosaea hohenbergiae sp. nov. and Carlosrosaea aechmeae sp. nov., two tremellaceous yeasts isolated from bromeliads in north-eastern Brazil. Int J Syst Evol Microbiol 67(6):1752–1757
Felix CR, Andrade DA, Almeida JH, Casanova Navarro HM, Fell JW, Landell MF (2020) Vishniacozyma alagoana sp. nov. a tremellomycetes yeast associated with plants from dry and rainfall tropical forests. Int J Syst Evol Microbiol. https://doi.org/10.1099/ijsem.0.004193
Fell JW, Statzell-Tallman A, Scorzetti G, Gutierrez MH (2011) Five new species of yeasts from fresh water and marine habitats in the Florida Everglades. Antonie Van Leeuwenhoek 99:533–549
Ferreira RB, Beard KH, Crump ML (2016) Breeding guild determines frog distributions in response to edge effects and habitat conversion in the Brazil’s Atlantic Forest. PLoS One 11(6):e0156781. https://doi.org/10.1371/journal.pone.01567811
Fonseca A, Inacio J (2006) Phylloplane yeasts. In: Peter G, Rosa CA (eds) Biodiversity and ecophysiology of yeasts. Springer-Verlag, Berlin
Formoso A, Heidrich D, Felix CR, Tenório AC, Leite BR et al (2015) Enzymatic activity and susceptibility to antifungal agents of Brazilian environmental isolates of Hortaea werneckii. Mycopat. 180:345–352
Frank JH, Lounibos LP (2009) Insects and allies associated with bromeliads: a review. Terr Arthropod Rev 1(2):125–153
Givnish TJ, Barfuss MHJ, Van Ee B, Riina R, Schulte K, Horres R et al (2014) Adaptive radiation, correlated and contingent evolution, and net species diversification in Bromeliaceae. Mol Phylogenet Evol 71(1):55–78
Gomes FCO, Safar SVB, Marques AR, Medeiros AO, Santos ARO, Carvalho C, Lachance MA, Sampaio JP, Rosa CA (2015) The diversity and extracellular enzymatic activities of yeasts isolated from water tanks of Vriesea minarum, an endangered bromeliad species in Brazil, and the description of Occultifur brasiliensis f.a., sp. nov. Antonie Van Leeuwenhoek 107(2):597–611. https://doi.org/10.1007/s10482-014-0356-4
Gomes FCO, Safar SVB, Santos ARO, Lachance MA, Rosa CA (2016) Kockovaella libkindii sp. nov., a yeast species isolated from water tanks of bromeliad. Int J Syst Evol Microbiol 66(12):5066–5069
Green MR, Sambrook J (2012) Molecular cloning: a laboratory manual. Cold Spring Harbor Laboratory Press, Cold Spring Harbor
Grondin E, Shum Cheong Sing A, Caro Y, Raherimandimby M, Randrianierenana AL, James S et al (2015) A comparative study on the potential of epiphytic yeasts isolated from tropical fruits to produce flavoring compounds. Int J Food Microbiol 203:101–108
Gunde-Cimerman N, Plemenitaš A (2006) Ecology and molecular adaptations of the halophilic black yeast Hortaea werneckii. Rev Environ Sci Biotechnol 5(2–3):323–331. https://doi.org/10.1007/s11157-006-9105-0
Hagler AN, Rosa CA, Morais PB, Mendonça-Hagler LC (1993) Yeasts and coliform bacteria of water accumulated in bromeliads of mangrove and sand dune ecosystems of southeast Brazil. Can J Microbiol 39(10):973–977
Imanishi Y, Jindamorakot S, Mikata K, Nakagiri A, Limtong S, Potacharoen W et al (2008) Two new ascomycetous anamorphic yeast species related to Candida friedrichii - Candida jaroonii sp. nov., and Candida songkhlaensis sp. nov.—isolated in Thailand. Antonie Van Leeuwenhoek 94(2):267–276
Inacio J, Pereira P, de Carvalho M, Fonseca A, Amaral-Collaco MT, Spencer-Martins I (2002) Estimation and diversity of phylloplane mycobiota on selected plants in a Mediterranean-type ecosystem in Portugal. Microb Ecol 44:344–353
Inácio J, Landell MF, Valente P, Wang PH, Wang YT, Yang SH, Manson JS, Lachance MA, Rosa CA, Fonseca A (2008) Farysizyma gen. nov., an anamorphic genus in the Ustilaginales to accommodate three novel epiphytic basidiomycetous yeast species from America, Europe and Asia. FEMS Yeast Res 8(3):499–508
Inácio J, Ludwig W, Spencer-Martins I, Fonseca A (2010) Assessment of phylloplane yeasts on selected Mediterranean plants by FISH with group- and species-specific oligonucleotide probes. FEMS Microbiol Ecol 71(1):61–72. https://doi.org/10.1111/j.1574-6941.2009.00784.x
Into P, Pontes A, Jacques N, Casaregola S, Limtong S, Sampaio JP (2018) Papiliotrema plantarum sp. nov., a novel tremellaceous sexual yeast species. Int J Syst Evol Microbiol 68:1937–1941
Into P, Pontes A, Sampaio JP, Limtong S (2020) Yeast diversity associated with the Phylloplane of corn plants cultivated in Thailand. Microorganisms 8(1):80
Isaeva OV, Glushakova AM, Garbuz SA et al (2010) Endophytic yeast fungi in plant storage tissues. Biol Bull Russ Acad Sci 37(1):26–34. https://doi.org/10.1134/S1062359010010048
Joly CA, Metzger JP, Tabarelli M (2014) Experiences from the Brazilian Atlantic Forest: ecological findings and conservation initiatives. New Phytol:459–473
Kembel SW, Mueller RC (2014) Plant traits and taxonomy drive host associations in tropical phyllosphere fungal communities. Botany. 92(4):303–311
Kemler M, Witfeld F, Begerow D, Yurkov A (2017) Phylloplane yeasts in temperate climates. In: Buzzini P, Lachance MA, Yurkov A (eds) Yeasts in natural ecosystems: diversity. Springer, Cham. https://doi-org-443.webvpn.fjmu.edu.cn/10.1007/978-3-319-62683-3_6
Khunnamwong P, Ribeiro JRA, Garcia KM, Hagler AN, Takashima M, Ohkuma M, Endoh R, Sugita T, Jindamorakot S, Limtong S (2017) Occultifur plantarum f.a., sp. nov., a novel cystobasidiomycetous yeast species. Int J Syst Evol Microbiol 67:2628–2633
Kumar S, Stecher G, Li M, Knyaz C, Tamura K (2018) MEGA X: Molecular Evolutionary Genetics Analysis across computing platforms. Mol Biol Evol 35:1547–1549
Kurtzman CP, Robnett CJ (1998) Identification and phylogeny of ascomycetous yeasts from analysis of nuclear large subunit (26S) ribosomal DNA partial sequences. Antonie Van Leeuwenhoek 98(183584):331–371
Lachance MA, Bowles JM, Starmer WT (2003) Geography and niche occupancy as determinants of yeast biodiversity: the yeast-insect-morning glory ecosystemof Kīpuka Puaulu, Hawai'i. FEMS Yeast Res 4(1):105–111. https://doi.org/10.1016/S1567-1356(03)00149-1
Landell MF et al (2010) Candida aechmeae sp. nov. and Candida vrieseae sp. nov., novel yeast species isolated from the phylloplane of bromeliads in Southern Brazil. Int J Syst Evol Microbiol 60(Pt 1):244–248
Landell MF, Brandão LR, Barbosa AC, Ramos JP, Safar SVB, Gomes FCO et al (2014) Hannaella pagnoccae sp. nov., a tremellaceous yeast species isolated from plants and soil. Int J Syst Evol Microbiol 64(PART 6):1970–1977
Landell MF, Brandão LR, Safar SVB, Gomes FCO, Félix CR, Santos ARO et al (2015) Bullera vrieseae sp. nov., a tremellaceous yeast species isolated from bromeliads. Int J Syst Evol Microbiol 65(8):2466–2471
Landell MF, Inácio J, Fonseca A, Vainstein MH, Valente P (2009) Cryptococcus bromeliarum sp. nov., an orange-coloured basidiomycetous yeast isolated from bromeliads in Brazil. Int J Syst Evol Microbiol 59(Pt 4):910–913
Landell MF, Mautone JN, Valente P (2006) Biodiversity of yeasts associated to bromeliads in Itapuã Park Viamão/RS. Biociencias. 14(2):144–149
Larsen CN, Krantz BA, Wilkinson KD (1998) Substrate specificity of deubiquitinating enzymes: ubiquitin C-terminal hydrolases. Biochemistry 37(10):3358–3368. https://doi.org/10.1021/bi972274d
Leitman P, Amorim AM, Sansevero JBB, Forzza RC (2015) Floristic patterns of epiphytes in the Brazilian Atlantic Forest, a biodiversity hotspot. Bot J Linn Soc 179(2009):587–601
Limtong S, Koowadjanakul N (2012) Yeasts from phylloplane and their capability to produce indole-3-acetic acid. World J Microbiol Biotechnol 28(12):3323–3335
Limtong S, Nasanit R (2017) Phylloplane yeasts in tropical climates. In: Yeasts in Natural Ecosystems: Diversity. Springer, Cham, pp 199–223
Limtong S, Kaewwichian R, Yongmanitchai W, Kawasaki H (2014) Diversity of culturable yeasts in phylloplane of sugarcane in Thailand and their capability to produce indole-3-acetic acid. World J Microbiol Biotechnol 30(6):1785–1796. https://doi.org/10.1007/s11274-014-1602-7
Lindow SE, Brandl MT (2003) Microbiology of the phyllosphere. Appl Environ Microbiol 69(4):1875–1883. https://doi.org/10.1128/aem.69.4.1875-1883.2003
Liu XZ, Wang QM, Göker M, Groenewald M, Kachalkin AV, Lumbsch HT et al (2015) Towards an integrated phylogenetic classification of the Tremellomycetes. Stud Mycol 81:85–147
Louca S, Jacques SMS, Pires APF, Leal JS, Srivastava DS, Parfrey LW et al (2016) High taxonomic variability despite stable functional structure across microbial communities. Nat Ecol Evol 1(Dec):1–12
Mautone JN, Landell MF, Fuentefria AM, Valente P (2010) Phylloplane yeasts as a source of industrially interesting enzymes. Braz J Biosci 8(9):169–173
Meneses DP, Gudiña EJ, Fernandes F, Gonçalves LRB, Rodrigues LR, Rodrigues S (2017) The yeast-like fungus Aureobasidium thailandense LB01 produces a new biosurfactant using olive oil mill wastewater as an inducer. Microbiol Res 204:40–47
Mestre LAM, Aranha JMR, Eser MLP (2001) Macroinvertebrate fauna associated to the bromeliad Vriesea inflata of the Atlantic Forest (Paraná State, southern Brazil). Braz Arch Biol Technol 44(1):89–94
Meyer KM, Leveau JHJ (2012) Microbiology of the phyllosphere: a playground for testing ecological concepts. Oecologia 168(3):621–629. https://doi.org/10.1007/s00442-011-2138-2
Mittelbach M, Yurkov AM, Nocentini D, Nepi M, Weigend M, Begerow D (2015) Nectar sugars and bird visitation define a floral niche for basidiomycetous yeast on the Canary Islands. BMC Ecol 15(1):2
Moubasher AH, Abdel-Sater MA, Zeinab SMS (2018) Diversity of floricolous yeasts and filamentous fungi of some ornamental and edible fruit plants in Assiut area, Egypt. Curr Res Environ Appl Mycol 8(1):135–161
Oksanen J, Blanchet FG, Friendly M, Kindt R et al (2019) Vegan: Community Ecology Package. R package version 2.5–6. https://CRAN.Rproject.org/package=vegan
Ortiz-Castellanos L, Ruiz-Herrera J (2015) Phylogenetic relationships of the wall-synthesizing enzymes of Basidiomycota confirm the phylogeny of their subphyla. Folia Microbiol 60(2):143–150
Pagani DM, Brandão LR, Santos ARO, Felix CR, Ramos JP, Broetto L et al (2016) Papiliotrema leoncinii sp. nov. and Papiliotrema miconiae sp. nov., two tremellaceous yeast species from Brazil. Int J Syst Evol Microbiol 66(4):1799–1806
Paulino GVB, Félix CR, Broetto L, Landell MF (2017) Diversity of culturable yeasts associated with zoanthids from Brazilian reef and its relation with anthropogenic disturbance. Mar Pollut Bull 123(1–2):253–260
Pereira GDA, Gomes GT, Rozolem AM, de Nóbrega GMDA, Barcellos FG, Rodrigues EP (2014) Isolation, identification and screening of hidrolytic enzymes producing phylloplane yeasts. BMC Proc 8(Suppl 4):P261. https://doi.org/10.1186/1753-6561-8-S4-P261
Peterson SW, Manitchotpisit P, Leathers TD (2013) Aureobasidium thailandense sp. nov. isolated from leaves and wooden surfaces. Int J Syst Evol Microbiol 63(PART2):790–795
Piatek M, Lutz M, Sousa FMP et al (2017) Pattersoniomyces tillandsiae gen. et comb. nov.: linking sexual and asexual morphs of the only known smut fungus associated with Bromeliaceae. Org Divers Evol 17:531–543
Poorter L, Bongers F, Aide TM, Almeyda Zambrano AM, Balvanera P, Becknell JM (2016) Biomass resilience of neotropical secondary forests. Nature. 530(7589):211–214
Prakash A, Randhawa HS, Khan ZU et al (2018) Environmental distribution of Cryptococcus species and some other yeast-like fungi in India. Mycoses. 61(5):305–313
RStudio Team (2020) RStudio: integrated development for R. RStudio, PBC, Boston, MA URL http://www.rstudio.com
Ruivo CCC, Lachance MA, Rosa CA, Bacci M, Pagnocca FC (2005) Candida bromeliacearum sp. nov. and Candida ubatubensis sp. nov., two yeast species isolated from the water tanks of Canistropsis seidelii (Bromeliaceae). Int J Syst Evol Microbiol 55(5):2213–2217
Safar SVB, Gomes FCO, Marques AR, Lachance MA, Rosa CA (2013) Kazachstania rupicola sp. nov., a yeast species isolated from water tanks of a bromeliad in Brazil. Int J Syst Evol Microbiol 63:1165–1168
Slifkin M (2000) Tween 80 opacity test responses of various Candida species. J Clin Microbiol 38(12):4626–4628. https://doi.org/10.1128/JCM.38.12.4626-4628.2000
Sørensen T (1948) A method of establishing groups of equal amplitude in plant sociology based on similarity of species content. K Dan Vidensk Selsk Biol Skr 4:1–34
Sousa FMP, Morais PB, Lachance MA, Rosa CA (2014) Hagleromyces gen. nov., a yeast genus in the Saccharomycetaceae, and description of Hagleromyces aurorensis sp. nov., isolated from water tanks of bromeliads. Int J Syst Evol Microbiol 64:2915–2919
Srisuk N, Nutaratat P, Surussawadee J et al (2019) Yeast communities in sugarcane phylloplane. Microbiology 88(3):353–369. https://doi.org/10.1134/S0026261719030135
Staden R, Beal KF, Bonfield JK (2000) The Staden Package (1998). In: Misener S, Krawetz SA (eds) Bioinformatics Methods and Protocols. Methods in Molecular Biology™, vol 132. Humana Press, Totowa, pp 115–130
Starmer WT, Lachance MA (2011) Chapter 6—yeast ecology. In: Kurtzman CP, Fell JW, Boekhout T (eds) The Yeasts, Fifth edn. Elsevier, pp 65–83 ISBN 9780444521491
Strauss MLA, Jolly NP, Lambrechts MG, van Rensburg P (2001) Screening for the production of extracellular hydrolytic enzymes by non-Saccharomyces wine yeasts. J Appl Microbiol 91:182–190
Tedersoo L, Bahram M, Polme S, Koljalg U, Yorou NS, Wijesundera R et al (2014) Global diversity and geography of soil fungi. Science 346(6213):1256688
Thakur S (2017) Study of phylloplane mycoflora of some selected medicinal plants. 8(2):1–7
Valente P, Boekhout T, Landell MF, Crestani J, Pagnocca FC, Sette LD et al (2012) Bandoniozyma gen. nov., a genus of fermentative and non-fermentative Tremellaceous yeast species. PLoS One 7(10):e46060
van Beilen JB, Li Z (2002) Enzyme technology: an overview. Curr Opin Biotechnol 13(4):338–344. https://doi.org/10.1016/s0958-1669(02)00334-8
Vaz ABM, Rosa LH, Vieira MLA, García V, Brandão LR, Teixeira LCRS, Moliné M, Libkind D, van Broock M, Rosa CA (2011) The diversity, extracellular enzymatic activities and photoprotective compounds of yeasts isolated in Antarctica. Braz J Microbiol 42(3):937–947
Vorholt JA (2012) Microbial life in the phyllosphere. Nat Rev Microbiol 10(12):828–840. https://doi.org/10.1038/nrmicro2910
White TJ, Bruns T, Lee S, Taylor J (1990) Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In: Innis MA, Gelfand DH, Sninsky JJ, White TJ (eds) PCR protocols a guide to methods and applications. Academic Press, London, pp 315–322
Williams KJ, Rosauer A, De Silva D, Mittermeier N, Bruce R et al (2011) Forests of East Australia: the 35th biodiversity hotspot. In: Zachos F, Habel J (eds) Biod Hotsp. Springer, Berlin, Heidelberg
Yurkov A (2017) Temporal and geographic patterns in yeast distribution. In: Buzzini P, Lachance MA, Yurkov A (eds) Yeasts in natural ecosystems: ecology. Springer, Berlin, pp 101–130
Yurkov A, Pozo MI (2017) Yeast community composition and structure. In: Buzzini P, Lachance MA, Yurkov A (eds) Yeasts in natural ecosystems: ecology. Springer, Berlin, pp 73–100
Yurkov A, Guerreiro MA, Sharma L, Carvalho C, Fonseca Á (2015) Multigene assessment of the species boundaries and sexual status of the Basidiomycetous yeasts Cryptococcus flavescens and C. terrestris (Tremellales). PLoS One 10(4):e0126996
Acknowledgments
The authors would like to thank the environmental entities responsible for issuing the authorizations to collect the material in the field, the Instituto Chico Mendes de Biodiversidade (ICMBio), the representatives of Parque Memorial Zumbi dos Palmares and Usina Caeté (Grupo Carlos Lyra) for the permissions, and the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq, number of grants 475378/2013-0, 408718/2013-7, and 311553/2018-4), Fundação de Amparo a Pesquisa de Alagoas (FAPEAL), and Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES). H.M.C.N. received a fellowship from PEC-PG/CNPq. C.R.F. and G.V.B.P. received a fellowship from CAPES. The authors also thank to Msc. Andressa Leticia Lopes for correcting and editing English.
Funding
This research was supported by the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq, number of grants 475378/2013-0, 408718/2013-7, and 311553/2018-4), Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES), and Fundação de amparo a Pesquisa de Alagoas (FAPEAL).
Author information
Authors and Affiliations
Contributions
H.M.C.N.: formal analysis, investigation, data curation, writing—original draft; C.R.F.: formal analysis, investigation, data curation, writing - original draft, visualization; G.V.B.P.: formal analysis, writing—original draft; J.H.A.: investigation, data curation; P.V.: supervision, writing—review and editing; M.F.L.: conceptualization, supervision, project administration, funding acquisition, writing—review and editing.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This article does not contain any studies with animals performed by any of the authors.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Code availability
Not applicable.
Additional information
Section Editor: Marc Stadler
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Supplementary Figure 1
(PNG 299 kb).
Supplementary Figure 2
(PNG 110 kb).
Supplementary Figure 3
(PNG 504 kb).
Supplementary Figure 4
(PNG 166 kb).
Supplementary Figure 5
(PNG 227 kb).
Supplementary Figure 6
(PNG 3774 kb).
Supplementary Table 1
(DOCX 81 kb).
Supplementary Table 2
(DOCX 15 kb).
Rights and permissions
About this article
Cite this article
Casanova Navarro, H.M., Félix, C.R., Paulino, G.V.B. et al. Richness and biotechnological potential of the yeast community associated with the bromeliad phylloplane in the Brazilian Neotropical Forest. Mycol Progress 19, 1387–1401 (2020). https://doi.org/10.1007/s11557-020-01631-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11557-020-01631-2