Abstract
The increased ultraviolet radiation (UV) due to the altered stratospheric ozone leads to multiple plant physiological and biochemical adaptations, likely affecting their interaction with other organisms, such as pests and pathogens. Arbuscular mycorrhizal fungi (AMF) and UV-B treatment can be used as eco-friendly techniques to protect crops from pests by activating plant mechanisms of resistance. In this study, we investigated plant (Lactuca sativa) response to UV-B exposure and Funneliformis mosseae (IMA1) inoculation as well as the role of a major insect pest, Spodoptera littoralis. Lettuce plants exposed to UV-B were heavier and taller than non-irradiated ones. A considerable enrichment in phenolic, flavonoid, anthocyanin, and carotenoid contents and antioxidant capacity, along with redder and more homogenous leaf color, were also observed in UV-B-treated but not in AMF-inoculated plants. Biometric and biochemical data did not differ between AMF and non-AMF plants. AMF-inoculated plants showed hyphae, arbuscules, vesicles, and spores in their roots. AMF colonization levels were not affected by UV-B irradiation. No changes in S. littoralis-feeding behavior towards treated and untreated plants were observed, suggesting the ability of this generalist herbivore to overcome the plant chemical defenses boosted by UV-B exposure. The results of this multi-factorial study shed light on how polyphagous insect pests can cope with multiple plant physiological and biochemical adaptations following biotic and abiotic preconditioning.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Insect-plant interactions are routed by a hierarchy of physical and chemical cues, and their full understanding represents a fascinating ecological challenge (Braga and Janz 2021). For instance, host plants can induce chemical and morphological responses to face insect attacks (Sharma et al. 2021). Although this appears to be a bi-directional relationship, numerous abiotic and biotic factors can mediate it, further complicating the interactions (Sharma et al. 2021). Many microorganisms (e.g., arbuscular mycorrhizal fungi, AMF) can stimulate plant growth by facilitating the absorption of nutrients, enhancing the efficient use of soil natural resources, and promoting plant resistance to biotic and abiotic factors (Smith and Read 2008). In addition, AMF can alter plant secondary metabolism, leading to higher synthesis of antioxidant metabolites (Agnolucci et al. 2020); as such, AMF are increasingly considered a biotechnological tool for the sustainable production of safe and healthy plant foods, especially horticultural crops (Zhu et al. 2022; Messa & Savioli 2021; Fusco et al. 2022). AMF-related effects on the plant are commonly beneficial for both the insect and the plant itself (Sharma et al. 2017; Yu et al. 2022). Plant quality improves as a food source for insects when plant nutrient content is increased (Vannette and Hunter 2011). On the other hand, AMF may induce plant resistance by priming the jasmonic acid-dependent plant responses to phytophagous insects and changing the concentration and composition of terpenoids (Barber et al. 2013; Sharma et al. 2017). Such modifications may alter the plant attractiveness to insects, as well as insect behavior (Agathokleous et al. 2017; Masui et al. 2021; Sharma et al. 2017).
High intensity UV radiation, in particular the UV-B component (280–315 nm), can have a strong impact on several morphophysiological, molecular, and biochemical traits of plants (Jaiswal et al. 2022; Pandey et al. 2022a, 2022b; Rai and Agrawal 2021, 2022). Therefore, plants have evolved a fine UV-B perception mechanism and transduction pathway (Kliebenstein et al. 2002; Rizzini et al. 2011) to avoid intracellular impairments. Such responses lead to the increased content of reactive oxygen species (ROS)-scavenging and UV-B absorbing compounds, such as phenolic compounds (Brown et al. 2005; Favory et al. 2009; Santin et al. 2019, 2021a; Takshak and Agrawal 2019; Volkova et al. 2022). In addition, several studies reported a UV-B-triggered modulation in the content of photosynthetic pigments, such as chlorophylls and carotenoids (Carletti et al. 2003; Jansen et al. 2008; Santin et al. 2018, 2021b; Schreiner et al. 2012). These mechanisms are now understood to occur within the context of hormesis, where mild sub-toxic stress driven by a mild elevation of ROS activate signaling pathways and initiate adaptive responses that allow plants to cope with and prevent further harmful stress (Agathokleous 2021; Erofeeva 2022; Moustakas et al. 2022; Volkova et al. 2022). Due to the strong health-promoting properties of the bioactive compounds enhanced by the UV-B exposure, UV-B radiation has gained great attention as a green technology to improve the nutraceutical quality of agricultural plants in the last decades (Neugart and Schreiner 2018; Schreiner et al. 2012). Application of UV-B has been observed to have a positive effect on, e.g., basil (Mosadegh et al. 2018; Nascimento et al. 2020), rice (Faseela and Puthur 2018), chili pepper (Dolzhenko et al. 2010), mung bean (Wang et al. 2017), and wheat (Chen et al. 2019). Besides, UV-B radiation impacts insect-plant interactions, directly by affecting herbivore behavior or indirectly by altering plant biochemistry and morphology (Bornman et al. 2019; Prieto-Ruiz et al. 2019). In addition, the use of light-emitting diode (LED) illumination in the horticultural field is constantly expanding. Due to their energy efficiency, low radiant heat and durability as well as the possibility of customization in terms of wavelength emission and spectral composition, LED light represents an eco-friendly and economically sustainable solution as artificial lighting source (Bourget 2008; Bantis et al. 2018).
In addition, the secondary metabolites are major drivers of plant–insect herbivore interactions too, and the effects of such hormetic priming on plant–insect interactions are poorly understood even though UV priming is among the most promising priming approaches (Christou et al. 2022). Recently, UV-B radiation has also evolved as an environment friendly technology, with the potential of improving crop protection against agricultural insect pests, mainly by boosting both constitutive and inducible plant defenses (Escobar-Bravo et al. 2021; Qi et al. 2018). Studies on the influence of UV-B radiation on the production of volatile compounds are quite scarce (Jaiswal and Agrawal 2021; Johnson et al. 1999). Moreover, the use of UV-B LED light for horticultural purposes is at its infancy, with most current studies involving the use of UV-B fluorescent tubes for UV-B treatments. However, LEDs represent a valuable option, compared to UV-B fluorescent tubes, considering their longer lifespan, the higher energy efficiency, the negligible heat loss, and the higher customizability in terms of power and wavelength.
It is urgently necessary to reduce pesticide use in agricultural settings to avoid adverse effects on human health and the environment, as well as the rapid emergence of resistance in targeted species (Pavela and Benelli 2016). As a result, developing novel and eco-friendly pest management tools through habitat manipulation is a worthwhile research endeavor. Previous research has shown that both plant UV-B light exposure and mycorrhizal symbiosis can influence the arthropod feeding activity (Barber et al. 2013; Qi et al. 2018). However, little has been done to shed light on the potential effects of the interaction between UV-B radiation and AMF colonization of horticultural crops and their key arthropod pests (Zeni et al. 2023). In this framework, one may question whether UV radiation exposure and mycorrhizal symbionts can boost plant tolerance to polyphagous insect pests attacking horticultural crops. Therefore, the present study aims at unraveling the role of the combination of plant above- and below ground treatments (UV-B exposure and mycorrhization) in the feeding activity of key arthropod pests. To this end, we evaluated biochemistry, morphology, and physiology of Lactuca sativa L. plants with or without AMF and exposed or not to UV-B radiation as well as the feeding behavior of larvae of Spodoptera littoralis (Boisduval) (Lepidoptera: Noctuidae), a highly polyphagous insect that attacks over 40 plant families. We hypothesized that UV-B priming and AMF inoculation could enhance leaf defense potential and improve the performance of plants under herbivory.
Materials and methods
Insects
The insects tested here were mass-reared at the Entomology Lab of the Department of Agriculture, Food and Environment (DAFE), University of Pisa (Italy), under controlled conditions [27 ± 1 °C, 75% R.H., and 16:8 (L:D)-h photoperiod]. Batches of S. littoralis eggs were placed on filter paper in a plastic container. Newly hatched larvae were gently transferred on a semi-synthetic bean-based (Sorour et al. 2011). The larval development on semi-synthetic diet takes 18–20 days and includes 6 larval stages. In our feeding bioassay, we used 3rd–4th instar larvae.
Plant and fungal material
Organic seeds of the red leaf lettuce (L. sativa L. var. crispa) cv. Red Salad Bowl were bought from Landen company (Blumen Group, Milan, Italy). The research was carried out at DAFE, University of Pisa. The seeds were sterilized in a 5% sodium hypochlorite solution for 15 min with magnetic stirring, and then rinsed thoroughly with distilled water. The seeds were then sown on moistened filter paper in plastic trays (25 × 40 cm, 2 seeds cm−2). The trays were placed in climate-controlled chambers and kept in the dark at 24 °C for 72 h, followed by 16:8 (L:D)-h photoperiod. Blue/red (1:2 ratio) and green (10%) LEDs (C-LED, Imola, Italy) provided photosynthetic active radiation (PAR), with a photosynthetic photon flux density (PPFD) of 225 ± 5 μmol m−2 s−1.
The AMF species Funneliformis mosseae (T.H. Nicolson & Gerd.) C. Walker & A. Schüßler, isolate IMA1, was used in the experiment. The AMF isolate was obtained from pot cultures in the DAFE Microbiology Laboratory’s collection. The fungal inoculum was grown in a greenhouse for 6 months on Trifolium alexandrinum L. as a host plant in a mixture (1:1 by volume) of sterilized soil and calcined clay (OILDRI Chicago, IL, USA). At harvest, roots were cut into approximately 1-cm fragments and mixed with the substrate to form a homogeneous crude inoculum mixture, which was then air-dried and stored until use. Prior to the experiment, the biological activity of the inoculum was assessed using the mycorrhizal inoculum potential (MIP) bioassay described in Njeru et al. (2014), and a level of 50–60% was considered optimal.
Mycorrhizal and UV-B treatments
Once the cotyledons were fully expanded (about 5 days after sowing), the sprouts were transferred to polystyrene plug trays (16 mL per cell, one sprout per cell) with a sterilized calcined clay as substrate. The substrate of half cells (120) was mixed (1:1) with F. mosseae IMA1 crude inoculum. To ensure a common AMF-associated microbiota to uninoculated control plants, the other half of the cells (120) received the same amount of sterilized crude inoculum (mock inoculum), and each cell received 2 mL of a filtrate obtained by sieving a mixture of mycorrhizal inocula through a 50-µm pore diameter sieve and a Whatman paper no. 1 (Whatman International Ltd, Maidstone, Kent (Koide and Li 1989).
The seedlings were irrigated twice a week with half-strength Hoagland’s nutrient solution (pH 6, 1.15 mS cm–1 electrical conductivity (EC)). Furthermore, plantlets were irrigated with distilled water (10 mL per pot) as needed. After 1 week in the growth chamber, mycorrhizal (+ M) and non-mycorrhizal (− M) plantlets were exposed or not to supplemental UV-B radiation for 2 weeks. UV-B radiation was provided by UV-B LEDs (High Power UV-B LTPL-G35UVB308GH, LITE-ON Technology, Inc., Taipei City, Taiwan) assembled by C-LED company (C-LED, Imola, Italy). The emission peak of the LEDs was 308 nm (half band width, 15 nm). The output power per LED was 62 mW, and the view angle was 120°. The UV-B irradiance at the top of the plants was 0.4 W m–2, and the treatment lasted 16 h per day (equivalent to a daily UV-B dose of 23 kJ m–2). Irradiance was measured using the spectrometer (FLAME-T-XR1-ES S/N: FLMT07829, Ocean Insight, Maybachstrasse 11, Ostfildern, D-73760 Germany) with fiber optics (QP400-1-UV-BX; Ocean Insight) and cosine corrector (CC-3-UV-S; Ocean Insight). During the treatment period and prior to sampling, both the + UV-B and − UV-B groups of plants were also exposed to PAR with a 16:8 (L:D)-h photoperiod, as indicated in the previous paragraph. Preliminary tests on lettuce plants were used to determine the UV-B dose. UV-B irradiation was carried out during the photoperiod’s 16-h light cycle, as previously described. To avoid the transfer of mycorrhizal fungi, the + M and − M plants in both the UV-B treatment and control chambers were placed in separate plastic trays. Two weeks after the start of the UV-B treatment (3 weeks after mycorrhizal inoculation), plants from all four groups (− M/ − UV-B; − M/ + UV-B; + M/ − UV-B; + M/ + UV-B; 60 plants per treatment) were randomly divided and sampled for further analysis.
Biometric indexes
Five different plants (biological replicates) per experimental condition were harvested, and the total number of fully expanded leaves per plant (n) and plant height (cm) were determined. In addition, same plants were weighed to measure the fresh (FW; g) and dry weight (DW; 60 °C until constant weight). DW/FW ratio was also calculated and expressed as a percentage.
Total phenolic, flavonoid, and anthocyanin extraction and determination
Total phenolics, flavonoids, and anthocyanins were measured in three separated groups of plants per treatment; each group consisted in five randomly selected freeze-dried plants (fifteen plants per group per treatment totally). Extraction was performed on 50 mg of freeze-dried sample using the method described by Tavarini et al. (2019) with few modifications. Briefly, samples were extracted with 1.5 mL of 80% methanol, and then sonicated for 30 min. After stirring for 30 min, the samples were centrifuged, and the supernatant was collected and stored at 4 °C. The pellet was subjected to a further extraction with 1 mL of 80% methanol, and the supernatants were combined and stored at 4 °C prior to the assays above.
The total phenolic content was determined using the Folin–Ciocalteau method (Borbalàn et al. 2003), with the absorbance at 750 nm through an Ultrospec 2100 pro-UV–vis spectrophotometer (Amersham Biosciences). The concentration of total phenolics was expressed as mg of gallic acid equivalents (GAE) g−1 FW.
Flavonoid concentration was measured, according to Kim et al. (2003), and the absorbance at 510 nm was recorded. The concentration of flavonoids was expressed as mg of catechin equivalents (CAE) g−1 FW. Commercial standards were used to create standard curves for total phenolic and flavonoid evaluation (Sigma-Aldrich Chemical Co., St. Louis, MO, USA).
Total anthocyanins were extracted and determined using the pH differential method described by Giusti and Wrolstad (2001). In brief, 50 mg freeze-dried samples were extracted in acidified methanol (1% HCl), and absorbance at 530 and 700 nm was measured. The following formula is used to calculate the final absorbance (Af) of the samples:
Total anthocyanin concentration was expressed as µg of cyanidin-3-O-glucoside (molar extinction coefficient 26,900 L cm−1 mol−1; molecular weight 449.2 g mol−1) equivalents (C3GE).
Total antioxidant activity evaluation
Total antioxidant activity was measured in freeze-dried phenolic extracts using the ABTS (2,2-azinobis (3-ethylbenzothiazoline-6-sulfonic acid) and the ferric reducing antioxidant power (FRAP) assays, as described by Re et al. (1999) and Benzie and Strain (1996), respectively. According to the ABTS assay, the absorbance was measured at 734 nm, and the antioxidant activity was expressed as μmol of Trolox equivalent antioxidant capacity (TEAC) g−1 FW. According to the FRAP assay, the absorbance was read at 593 nm, and the antioxidant activity was expressed as μmol of Fe (II) g−1 FW. Through the calculation of standard curves, the respective commercial standards (Trolox and FeSO4, considering the ABTS and FRAP assays, respectively; Sigma-Aldrich Chemical Co., St. Louis, MO, USA) were used in both antioxidant activity determinations.
Chlorophyll and carotenoid determination
Chlorophylls a and b and total carotenoids were extracted and quantified spectrophotometrically (Ultrospec 2100 pro-UV–vis spectrophotometer, Amersham Biosciences) from three groups of five plants per group, following the method reported by Wellburn (1994) with few modifications. In brief, 150-mg samples were homogenized with 80% (w/v) cold acetone before being centrifuged (5500 × g per 5 min at 4 °C), and the supernatant was collected. A second volume of 80% cold acetone was added to the pellet, centrifuged (5500 × g per 5 min at 4 °C), and the resulting supernatant was combined with the first. This procedure was repeated until the supernatant after centrifugation was clear. The absorbance of the combined supernatant was read at 663, 648, and 470 nm.
Color measurement
Color was measured on three different fully expanded leaves per plant, five plants per experimental condition, using a Konica Minolta CR-600 portable colorimeter (Holdings, Inc., Osaka, Japan). Color was determined using the CIELab system (CIE 1977), where L* (lightness), a* (redness), and b* (yellowness) were used to calculate chroma (C*; (a*2 + b*2)1/2) and hue angle (H*; tan−1 (b*/a*)) indexes (Priolo et al. 2000). Measurements were conducted in an 8-mm area diameter, specular component included, and 0% UV, D65 standard illuminant, observer angle 10°, and zero and white calibration.
Analyses of AMF root colonization
For each treatment, the percentage of mycorrhizal colonization on the total root system of 12 plants was calculated. Following Phillips and Hayman (1970) method, each root system was rinsed with tap water, clarified, and dyed with 0.05% Trypan blue in lactic acid rather than phenol. The grid line intersection method was used to calculate the percentage of colonized roots length (Giovannetti and Mosse 1980). To check for the presence of intraradical AM fungal structures (e.g., appressoria and arbuscules), roots were mounted on slides with lactic acid and examined using a Reichert-Jung (Wien, Austria) Polyvar light microscope.
Feeding assay with Spodoptera littoralis larvae
To determine the feeding preference of S. littoralis larvae towards different treated/untreated lettuce plants, a no-choice test was performed following the method of Vandenbussche et al. (2018) with slight modifications. A 3rd–4th instar larva was isolated and gently transferred to a Petri dish containing a leaf of treated/untreated lettuce. To absorb the excess moisture released by the leaf, a filter paper was placed on the bottom of the Petri dish. The Petri dishes were stored in the dark to avoid the larvae visual orientation. The acceptability of the leaves to the larvae was evaluated by noting the proportion of consumed leaves after 18 h. A total of 40 leaves were used per treatment, with two leaves chosen at random for each plant. To evaluate the amount of consumed leaf, a picture of each leaf has been taken before the introduction into the Petri dish and after 18 h, using a Nikon D5300 digital camera (Tokyo, Japan). The leaves were placed on a square of white paper of a known area (10 cm × 10 cm). Each picture was cut out to the size of a square and converted to an 8-bit black-and-white image. ImageJ software was used to calculate the size of the leaf area (black area) in relation to the paper square (white area). Therefore, the percentage of the consumed leaf was assessed by comparing the initial leaf area to the remaining leaf area after 18 h of larval feeding.
Statistical analysis
Differences in the biochemical parameters analyzed between the different groups of plants, considering both the mycorrhization (− M and + M) and the UV-B treatment (+ UV-B and − UV-B) were assessed with a two-way ANOVA followed by post hoc Tukey–Kramer test (p < 0.05) to evaluate significant interactions, and data are reported as mean ± standard error (SE). JMP software (JMP®, Version 16. SAS Institute Inc., Cary, NC, 1989–2021) was used to perform both the two-way ANOVA and a multivariate statistic with an unsupervised approach, a principal component analysis (PCA), and a two-way hierarchical clustering analysis (HCA). PCA combines all the n measured variables simultaneously (biometric, biochemical, and color parameters investigated in this study) to display possible clustering patterns according to newly generated p variables (p < n), the so-called principal components (PCs). In this study, the first two PCs, which explain most of the differences, were considered. The HCA (Euclidean distance, Ward’s linkage) was used to underline the similarity among the groups and the parameters considered. Data of mycorrhizal colonization were analyzed by independent Student’s t-test to assess the effect of UV-B treatment on inoculated plants, using SPSS statistical package v. 23.0. GraphPad Prism (v. 9.5.1, GraphPad Software, San Diego, California, USA) was used to analyse data collected from the no-choice assays on S. littoralis. The data were previously transformed with arcsine√ to evaluate their distribution. As the goodness-of-fit test evaluating the data distribution outlined that they were not normally distributed (Shapiro–Wilk test, p < 0.01), differences in larval acceptance of leaves were analyzed using the Kruskal–Wallis test. A probability level of p < 0.05 was used for the significance of differences between means.
Results
Mycorrhizal status of experimental plants
Lettuce plants inoculated with F. mosseae IMA1, collected 21 days post-inoculation, independently of UV-B treatment, showed in their roots the typical symbiotic structures of AMF, such as hyphae, arbuscules, vesicles, and spores, detected under the optical microscope (Fig. 1).
Light photomicrographs of fungal structures formed by Funneliformis mosseae IMA1 on the roots of Lactuca sativa L. (+ M) exposed or not to UV-B light (+ UV-B or − UV-B). a, b. Fungal extraradical and intraradical hyphae and appressoria formed on the root surface (scale bars: a, 30 µm; b, 20 µm); c, d. Arbuscules produced within cortical root cells (scale bar 15 µm)
On the contrary, control plants did not show any colonization, as expected, since they were mock inoculated. UV-B treatment did not significantly affect AMF colonization in inoculated plants (t = 1.66, p = 0.11). Mycorrhizal root length independent of UV-B treatment was 27.7 ± 9.3% (mean ± SD).
Biometric indexes and dry matter content
Weight and length of the plants, number of leaves per plant, and dry matter content were evaluated (Table 1). The interaction between UV-B exposure and mycorrhization did not significantly affect any of the aforementioned traits. Considering only the mycorrhization, − M and + M plants did not show any significant difference in the parameters analyzed, except for the plant length; − M plants were on average 6.8% higher than the + M plants. UV-B radiation was effective in increasing both the plant weight and length (by 26.7% and 20.8%, respectively), while no significant differences were observed in terms of the number of leaves and dry matter percentage between − UV-B and + UV-B plants.
Total phenolic, flavonoid, anthocyanin contents and antioxidant capacity
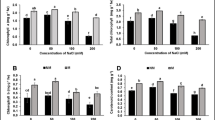
The interaction between the mycorrhization and UV-B treatment did not produce statistically significant results in terms of total phenolics, flavonoids, antioxidant capacity, and total anthocyanins. Mycorrhizal and non-mycorrhizal lettuce plants did not significantly differ in total phenolics, flavonoids, and total anthocyanin contents, as well as in the antioxidant capacity measured with either ABTS or FRAP assay (Fig. 2). Conversely, UV-B exposure enhanced the content of these secondary metabolites. Specifically, lettuce plants exposed to UV-B radiation displayed a 53, 37, and 102% higher content of total phenolics, flavonoids, and anthocyanins, respectively. Similarly, the antioxidant activity of UV-B irradiated plants was significantly higher (28% in ABTS assay and 62% in FRAP assay), compared to the non-irradiated plants.
Determination of (a) total phenolics; (b) flavonoids; antioxidant capacity measured through (c) ABTS, and (d) FRAP assays; (e) total anthocyanins of mycorrhizal (+ M) or non-mycorrhizal (− M) lettuce plants, UV-B-treated (+ UV-B) or untreated (− UV-B). The two-way ANOVA results are shown in the box below each histogram. n.s., not significant. According to two-way ANOVA, there were no significant interaction effects (n = 3, p > 0.05)
Chlorophyll and carotenoid content
The interaction between UV-B exposure and mycorrhization did not significantly impact the chlorophylls a/b ratio, the sum of chlorophylls a + b or the total carotenoid concentrations (Table 2). No statistically differences were found between mycorrhizal and non-mycorrhizal plants in terms of all the photosynthetic pigments measured. However, UV-B radiation increased the concentration of total carotenoids by 45%, compared to the non-irradiated plants. No significant differences between UV-B irradiated and unirradiated plants were observed considering the chlorophylls a/b ratio and the sum of chlorophylls a + b.
Color determination
UV-B exposure was the only factor significantly affecting the color of lettuce leaves (Table 3). Particularly, UV-B-treated plants showed 46, 44, and 45% smaller values of lightness (L*) and blue-yellow (b*) coordinates, and hue angle (H*), respectively, and 135% larger values of red-green (a*) coordinate. The mycorrhization and the interaction of mycorrhization and UV-B exposure were ineffective in significantly changing the aforementioned color parameters. To better visualize the clustering pattern according to the L*, a*, and b* coordinates, a 3D scatter chart was created (Fig. 3). The two main clusters detected in Fig. 3 corresponded to − UV-B (in the upper right portion of the space) and + UV-B (in the lower-left portion of the space) groups, regardless of the mycorrhization. Indeed, no clusters could be identified according to − M and + M plants, confirming that the mycorrhization did not modify the color parameters studied.
3D scatter chart setting L*, a*, and b* as coordinates, according to the CIE L*a*b* system. Different symbols refer to individual lettuce plants from the different groups referred to the mycorrhizal (+ M) or non-mycorrhizal (− M) lettuce plants, treated with UV-B radiation (+ UV-B) or not (− UV-B). Lightness (L*), redness (a*), and yellowness (b*) values for each plant are the mean of three independent measurements on three fully expanded leaves
Feeding assay with Spodoptera littoralis larvae
Since UV-B exposure increased the contents of total phenolics, flavonoids, and anthocyanins, we evaluated whether these conditions could influence the feeding preference of S. littoralis larvae. Caterpillars fed with treated lettuce plants (i.e. − M/ + UV-B; + M/ − UV-B; + M/ + UV-B) behave similarly to caterpillars fed with control plants (i.e. − M/ − UV-B) (Kruskal–Wallis, χ2 = 1.49, d.f. = 3, p = 0.68). In addition, the combination of both UV-B and AMF treatments did not significantly affect S. littoralis feeding behavior (Fig. 4). These results suggested that AMF inoculation and UV-B radiation had no indirect effects on the caterpillars.
Boxplot showing the percentage of leaf area (cm2) consumed by larvae of Spodoptera littoralis in differently treated lettuce plants. + M/ + UV-B = lettuce plants exposed to UV-B radiation and inoculated with the arbuscular mycorrhizal symbiont Funneliformis mossae; − M/ + UV-B = UV-B-exposed plants not inoculated with F. mossae; + M/ − UV-B = plants inoculated with F. mosseae and not exposed to UV-B; − M/ − UV-B = untreated lettuce plants, unexposed and not mycorrhizal inoculated (control). Each box plot indicates the median (lower, upper quartile and extreme values, outliers); n.s., not significant (Kruskal–Wallis test, p > 0.05)
Discussion
UV-B and mycorrhizal effect on lettuce plants
The potential of UV-B radiation and/or the use of AMF inocula to enhance the nutraceutical value of agricultural plants by increasing the content of health-promoting secondary metabolites has been investigated in several species, such as flaxseed (Santin et al. 2022), basil (Mosadegh et al. 2018; Nascimento et al. 2020, Battini et al. 2016), broccoli (Mewis et al. 2012; Moreira-Rodríguez et al. 2017a, 2017b), wheat (Chen et al. 2020), rice (Faseela and Puthur 2018), lettuce (Baslam et al. 2013; Avio et al. 2017), and others (Santin et al. 2021a, 2021b; Avio et al. 2018; Agnolucci et al. 2020). However, many studies are conducted using UV-B lamps as a light source (Assumpção et al. 2019; Aksakal et al. 2017; Basahi et al. 2014; Esringu et al. 2016; Lee et al. 2021; Park et al. 2007; Rajabbeigi et al. 2013; Rodriguez et al. 2014; Zhang et al. 2019), but research employing UV-B LEDs is scanty.
In this study, the lettuce plants exposed to UV-B from LED sources were 26.7% heavier and 20.8% taller than their control counterparts, suggesting a positive role of the irradiation on such biometric indexes. This observation contrasted with that of Tsormpatsidis et al. (2008), whose lettuce plants were cultivated in tunnels with or without UV (280–400 nm)-blocking filters. The authors found a lower vegetative growth (in terms of dry weight and leaf number) with the progressive increase in UV proportion. Similar to Tsormpatsidis et al. (2008), other studies reported a reduction in fresh and/or dry weight in UV-exposed lettuce (16–18 μmol m−2 s−2), although they used different lettuce cultivars (Diaz et al. 2006). UV-B irradiation negatively affected plant growth also in other plants of food interest, such as basil (18.7 kJ m−2 h−1), potato (10 kJ m−2 d−1), flaxseed 1.33 (W m−2), radish 7.2 (kJ m−2 d−1), rice (15.7 kJ m−2), barley (0.60–2.30 W m−2), bean (0.60–2.30 W m−2), and others (Chen et al. 2020; Dou et al. 2019; Santin et al. 2022; Singh et al. 2011; Teramura et al. 1991; Tevini et al. 1981). UV-B-related reduction in plant growth might be due to impairments in the cell cycle caused by DNA photo dimers (Biever et al. 2014; Yadav et al. 2020), and the likely damages to the photosynthetic apparatus and the consequent photosynthetic processes (Cuzzuol et al. 2020; Kosobryukhov et al. 2020; Piccini et al. 2020). In the current study, the increase of weight and height in the UV-B-exposed plants might be due to the lower UV-B irradiance compared to other studies, which might have lessened the appearance of UV-B-related stress-like symptoms. Indeed, in the present work, lettuce plants were exposed to low, ecologically relevant UV-B doses; therefore, it is reasonable to assume that the irradiation provided did not induce significant impairments in the plant growth and development, as conversely observed in other studies.
It is widely known from the literature that UV-B exposure can be associated with increased ROS levels, with consequent oxidative damage in plants (Gao and Zhang 2008). However, in case of moderate irradiation, plants can control oxidative stress by ROS-scavenging mechanisms that can prime plants, thus activating their pathways of defense and protecting against both abiotic and biotic stresses (Christou et al. 2022; Volkova et al. 2022). These indicate the hormetic-biphasic property of radiation and numerous chemical agents, with this dose-dependency explaining the contradictory findings between studies (Christou et al. 2022; Volkova et al. 2022). Recently, UV-B-induced oxidative stress was studied through histochemical detection of hydrogen peroxide and lipid peroxidation, as well as defense-related callose deposition in lettuce plants (Zeni et al. 2023); the authors also showed that the UV-B-induced stress was partially mitigated by the presence of AMF. Herein, the UV-B-triggered increase in the content of total phenolics, flavonoids, anthocyanins, as well as the higher antioxidant capacity, is in accordance with the results reported by Aksakal et al. (2017) and Esringu et al. (2016), who found that a 12- and 18-h UV-B irradiation (3.3 W m−2) effectively enhanced the contents of total phenolics and the antioxidant activity in lettuce seedlings, respectively. Similarly, a study carried out on the same lettuce cultivar (Red Salad Bowl) subjected to a 2-week UV-B radiation exposure (1 h daily, 1.69 W m−2) found a significantly higher flavonoid concentration. The ability of UV-B radiation to increase the content of phenolic compounds, particularly flavonoids, is mainly due to the activation of the UV-B photoreceptor UVR8, whose signaling cascade results in the upregulation of genes involved in the phenylpropanoid pathway, as observed in Arabidopsis (Heijde and Ulm 2012; Stracke et al. 2010) and in many other plant species (Giuntini et al. 2008; Hu et al. 2020; Santin et al. 2019, 2021b; Sheng et al. 2018; Ubi et al. 2006). The higher UV-B-triggered phenolic compounds, and consequently an enhanced antioxidant capacity, as acclimation response towards high UV-B conditions (Cloix et al. 2012; Rizzini et al. 2011) is due to the ROS-scavenging potential of such phytochemical, which can effectively prevent eventual damages to macromolecules by neutralizing the likely UV-B-induced ROS (Czégény et al. 2016; Hideg et al. 2013). In our study, we also found a significantly higher (+ 102%) concentration of total anthocyanins in the UV-B-treated plants, accompanied with a significantly redder and more homogeneous color of the leaves, as resulted from the parameters according to the CIE L*a*b* system. An enhanced content of anthocyanins was also observed by Assumpção et al. (2019), who reported a 101.35% increase of cyanidin glucoside in the UV-B-treated lettuce plants (cv. Red Salad Bowl). In line with our results, Park et al. (2007) reported that a UV-B irradiation (5-min pulse daily, 10 days totally, 0.26 kJ m−2 d−1) was effective to develop a red coloration in the leaves or red lettuce, likely due to a transcriptional activation of several flavonoid-related genes. A UV-B-induced activation of some anthocyanin biosynthetic genes was observed also with a transcriptome analysis by another study (Zhang et al. 2019), indicating that the accumulation of anthocyanin pigments in lettuce leaves occurs via transcriptional regulation through the UVR8 signaling pathway. Contrarily to our observations, Rajabbeigi et al. (2013) did not find any significant change in terms of total phenolic and anthocyanin concentration of lettuce in response to the UV-B treatment. However, the UV-B irradiation described by the authors was conducted with a much higher UV-B fluence rate (8.2 W m−2) and in a shorter time (5 days), indicating also a much greater dose rate; therefore, the biochemical effects triggered might be considerably different, since they are strictly dependent to both the UV-B irradiation and time of exposure (Jenkins 2017). Besides, the lettuce cultivar used (L. sativa var. capitata cv. Teodore RZ®) was different from that of our study, and physiological and biochemical responses to UV-B exposure are species- and cultivar-dependent (Rajabbeigi et al. 2013). Also, a UV-B exposure (10 h daily, 10 kJ m−2 d−1) of lettuce plants cv. Romaine was ineffective in increasing flavonoid content, although anthocyanins and total phenolics were significantly enhanced by the UV-B irradiation (Basahi et al. 2014). Photosynthetic pigments of lettuce plants were also found to be affected by the UV-B treatment in the present study. Particularly, total carotenoid concentration was increased in the UV-B-treated plants, regardless the mycorrhization, while no changes were found in the content of chlorophyll a + b or their ratio. Our findings partially agreed with the findings of Lee et al. (2021), who treated lettuce plants of New Red Fire and Two Star cultivars with 5 days of UV-B (24 h daily; 1.97 W m−2). The authors found no changes in chlorophyll a concentration in the New Red Fire cultivar, while both chlorophylls a and b were unaltered due to the irradiation in Two Star cultivar. Similarly, Li and Kubota (2009) did not find any modification in the chlorophyll content of lettuce (cv. Red Cross) plants grown under supplemental UV-B irradiation, while a significant increase in xanthophyll and β-carotene was detected. Consistent results were obtained also when lettuce was grown in boxes covered with either UV-B transparent or UV-B blocking films. Krizek et al. (1998) found that chlorophyll a and b contents were unchanged between lettuce plants (New Red Fire lettuce cultivar) receiving or not the UV-B solar component. Similar to our findings, Assumpção et al. (2019) reported no differences in chlorophyll and carotenoid concentration between UV-B-treated and untreated plants in the same cultivar (Red Salad Bowl) as in the present work. UV-B-induced increase of carotenoids, as reported in the present manuscript, was found also in other plant species, e.g., broccoli (Moreira-Rodríguez et al. 2017a, b), tomato (Perez et al. 2008), and canola (Qaderi et al. 2010). However, UV-B-related modulation of photosynthetic pigments is also genotype-dependent (Santin et al. 2021a; Schreiner et al. 2012). Carotenoids were found to increase by various stressors other than radiation and act as precursors of abscisic acid and some volatiles, among others, and are important for plant–insect interactions (Agathokleous 2021).
In this work, no changes in biometric and biochemical parameters were observed in mycorrhizal lettuce plants, except for a reduction in plant length. Arbuscular mycorrhizal symbiosis generally enhances plant growth; nevertheless, a reduction in plant length during symbiosis establishment may be linked to a modulation of hormonal balance during the development of fungal colonization (Liao et al. 2018). Here, colonization levels (approximately 30%) were similar to or even higher than those observed in other studies in the roots of different lettuce cultivars (Baslam and Goicoechea 2012; Avio et al. 2017). However, the sampling time (3 weeks post-inoculation), was not sufficient to detect differences in biochemical parameters between mycorrhizal and control plants. Most studies analyzing antioxidant compounds in mycorrhizal lettuce were carried out 7–8 weeks post-inoculation (Avio et al. 2017; Baslam et al. 2011a, 2011b, 2013; Goicoechea et al. 2015), when the symbiosis is generally well established with marked effects on plant secondary metabolism. Baslam et al. (2013) demonstrated that mycorrhizal inoculation increased not only lettuce growth, but also chlorophyll and/or carotenoid contents, particularly in the leaves that were most exposed to light, at such a sampling time. However, as observed in other food plants, a differential modulation of the expression patterns of genes encoding for key enzymes involved in secondary metabolite production cannot be ruled out, even 3 weeks after inoculation (Vangelisti et al. 2018). The statistically not significant results in terms of antioxidant activity, total phenolics, flavonoids, anthocyanins, and photosynthetic pigments between mycorrhizal and not-mycorrhizal lettuce plants used in the present work may be also explained by the lettuce cultivar used (Red Salad Bowl), which belongs to L. sativa var. crispa. Indeed, most data were obtained from cultivars, such as Batavia Rubia Munguía, Maravilla de Verano, Cogollos de Tudela, belonging to different botanical varieties, capitata or longifolia. For example, anthocyanin and carotenoid concentrations were usually shown to increase in the leaves of such cultivars (Baslam et al. 2011a, 2013; Goicoechea et al. 2015; Avio et al. 2018). Conversely, no effects on the enhancement of total phenolic concentration were observed in diverse investigations (Baslam et al. 2011a, 2011b, 2013), except for leaves of the cv. Batavia Rubia Munguía under optimal irrigation, in association with AMF (Baslam and Goicoechea 2012). The only study using two L. sativa var. crispa cultivars (Eluarde and Panisse) showed an increase of phenolics and antioxidant activity in the leaves of plants inoculated with different AMF isolates (Avio et al. 2017). Such data confirm that the production of antioxidant compounds is modulated by both plant genotype and AMF identity (Avio et al. 2018; Fusco et al. 2022).
UV-B and mycorrhizal effects on caterpillar feeding behavior
Insect-plant interactions result in a wide range of outcomes depending on a variety of both abiotic and biotic factors. For instance, both UV-B treatment and AMF inoculation have been observed to stimulate plant defense mechanisms by impacting plant physiology and biochemistry (Qi et al. 2018; Zeni et al. 2023). All these changes can affect the following trophic levels, such as the interaction between treated plants and herbivore insects (Gange 2007; Qi et al. 2018). Interestingly, no treatment alone or in combination affected the food preferences of S. littoralis larvae. Concerning the UV-B treatment alone, our results are consistent with those reported by Vandenbussche et al. (2018). Indeed, S. littoralis caterpillars performed equally well when fed with UV-B-treated plant material as when fed with not UV-B-treated ones. This occurred despite an increase in plant antioxidant capacity due to a higher total phenolic and flavonoid contents. As reported by Qi et al. (2018) in a study on Spodoptera litura (Fabricius) (Lepidoptera: Noctuidae) feeding activity, the UVR8-related downstream responses are not necessarily associated with plant resistance to chewing insects. Other studies reported the marginal effect of UV-B treatment on different Spodoptera species (Lindroth et al. 2000; Wargent and Jordan 2013). Our findings support the hypothesis that generalist herbivores, such as lepidopteran caterpillars or polyphagous aphids [e.g., Myzus persicae (Sulzer)] (Zeni et al. 2023), have evolved the ability to withstand the chemical defense mechanisms of a wide range of plant species. However, it is important to consider that higher UV-B doses than the one used in this work could further enhance the biochemical defenses in terms of phenolic accumulation, without impairing plant growth and development. Therefore, deeper investigations are encouraged to study the effects of stronger UV-B irradiation on the feeding behavior of such generalist herbivores. As to the effects of AMF on S. littoralis feeding behavior, we can state that our results fit the analysis conducted by Heinen et al. (2018). According to their literature review, 75% of relevant papers reported no effect, and 25% reported negative effects of AMF on generalist chewing herbivores like S. littoralis larvae (Gange and West 1994; Vicari et al. 2002). In conclusion, the effects of UV-B exposition and AMF inoculation on foraging S. littoralis caterpillars may be highly dependent on the degree of specialization of the insect species. Furthermore, the responses might also depend on the plant developmental stage and on the degree of insect adaptation to variation in host plant quality due to the treatments.
Conclusions
Overall, UV-B irradiation priming over AMF inoculation of the most suitable plant genotype may represent a promising approach to increase the nutraceutical and commercial quality of lettuce plants. The enhanced chemical defenses of plants did not impact the feeding behavior of S. littoralis larvae, probably due to (i) the generalist feeding traits of such herbivores, (ii) the mild UV-B treatments, and (iii) the limited time for the induction of mycorrhizal effects on plant secondary metabolism (see also Zeni et al. 2023). Therefore, further investigations are needed to assess whether different doses of UV-B/AMF and exposure durations (smaller or greater) that increase plant antioxidant defenses within a hormetic framework may alter the feeding behavior of oligophagous insect pests.
Data availability
Data are available from the corresponding author at reasonable request.
References
Agathokleous E (2021) The rise and fall of photosynthesis: hormetic dose response in plants. J for Res 32:789–803. https://doi.org/10.1007/s11676-020-01252-1
Agathokleous E, Sakikawa T, Abu ElEla SA, Mochizuki T, Nakamura M, Watanabe M, Kawamura K, Koike T (2017) Ozone alters the feeding behavior of the leaf beetle Agelastica coerulea (Coleoptera: Chrysomelidae) into leaves of Japanese white birch (Betula platyphylla var. japonica). Environ Sci Pollut Res 24:17577–17583. https://doi.org/10.1007/s11356-017-9369-7
Agnolucci M, Avio L, Palla M, Sbrana C, Turrini A, Giovannetti M (2020) Health-promoting properties of plant products: the role of mycorrhizal fungi and associated bacteria. Agronomy 10:1864. https://doi.org/10.3390/agronomy10121864
Aksakal O, Tabay D, Esringu A, Icoglu Aksakal F, Esim N (2017) Effect of proline on biochemical and molecular mechanisms in lettuce (Lactuca sativa L.) exposed to UV-B radiation. Photochem Photobiol Sci 16:246–254. https://doi.org/10.1039/c6pp00412a
Assumpção CF, Assis RQ, Hermes Poletto VS, Castagna A, Ranieri A, Neugart S, de Oliveira Rios A (2019) Application of supplemental UV-B radiation in pre-harvest to enhance health-promoting compounds accumulation in green and red lettuce. J Food Process Preserv 43:e14213. https://doi.org/10.1111/jfpp.14213
Avio L, Sbrana C, Giovannetti M, Frassinetti S (2017) Arbuscular mycorrhizal fungi affect total phenolics content and antioxidant activity in leaves of oak leaf lettuce varieties. Sci Hortic 224:265–271. https://doi.org/10.1016/j.scienta.2017.06.022
Avio L, Turrini A, Giovannetti M, Sbrana C (2018) Designing the ideotype mycorrhizal symbionts for the production of healthy food. Front Plant Sci 9:1089. https://doi.org/10.3389/fpls.2018.01089
Bantis F, Smirnakou S, Ouzounis T, Koukounaras A, Ntagkas N, Radoglou K (2018) Current status and recent achievements in the field of horticulture with the use of light-emitting diodes (LEDs). Sci Hortic 235:437–451. https://doi.org/10.1016/j.scienta.2018.02.058
Barber N, Kiers ET, Hazzard R, Adler L (2013) Context-dependency of arbuscular mycorrhizal fungi on plant-insect interactions in an agroecosystem. Front Plant Sci 4:338. https://doi.org/10.3389/fpls.2013.00338
Basahi JM, Ismail IM, Hassan IA (2014) Effects of enhanced UV-B radiation and drought stress on photosynthetic performance of lettuce (Lactuca sativa L. Romaine) plants. Annu Res Rev Biol 1739–1756. https://doi.org/10.9734/ARRB/2014/6638
Baslam M, Goicoechea N (2012) Water deficit improved the capacity of arbuscular mycorrhizal fungi (AMF) for inducing the accumulation of antioxidant compounds in lettuce leaves. Mycorrhiza 22:347–359. https://doi.org/10.1007/s00572-011-0408-9
Baslam M, Garmendia I, Goicoechea N (2011a) Arbuscular mycorrhizal fungi (AMF) improved growth and nutritional quality of greenhouse-grown lettuce. J Agric Food Chem 59:5504–5515. https://doi.org/10.1021/jf200501c
Baslam M, Pascual I, Sánchez-Díaz M, Erro J, García-Mina JM, Goicoechea N (2011b) Improvement of nutritional quality of greenhouse-grown lettuce by arbuscular mycorrhizal fungi is conditioned by the source of phosphorus nutrition. J Agric Food Chem 59:11129–11140. https://doi.org/10.1021/jf202445y
Baslam M, Garmendia I, Goicoechea N (2013) The arbuscular mycorrhizal symbiosis can overcome reductions in yield and nutritional quality in greenhouse-lettuces cultivated at inappropriate growing seasons. Sci Hortic 164:145–154. https://doi.org/10.1016/j.scienta.2013.09.021
Battini F, Bernardi R, Turrini A, Agnolucci M, Giovannetti M (2016) Rhizophagus intraradices or its associated bacteria affect gene expression of key enzymes involved in the rosmarinic acid biosynthetic pathway of basil. Mycorrhiza 26:699–707. https://doi.org/10.1007/s00572-016-0707-2
Benzie IF, Strain JJ (1996) The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: the FRAP assay. Anal Biochem 239:70–76. https://doi.org/10.1006/abio.1996.0292
Biever JJ, Brinkman D, Gardner G (2014) UV-B inhibition of hypocotyl growth in etiolated Arabidopsis thaliana seedlings is a consequence of cell cycle arrest initiated by photodimer accumulation. J Exp Bot 65:2949–3296. https://doi.org/10.1093/jxb/eru035
Borbalàn AMA, Zorro L, Guillen DA, Barroso CG (2003) Study of the polyphenol content of red and white grape varieties by liquid chromatography–mass spectrometry and its relationship to antioxidant power. J Chromatogr A 1012:31–38. https://doi.org/10.1016/S0021-9673(03)01187-7
Bornman JF, Barnes PW, Robson TM, Robinson SA, Jansen MAK, Ballaré CL, Flint SD (2019) Linkages between stratospheric ozone, UV radiation and climate change and their implications for terrestrial ecosystems. Photochem Photobiol Sci 18:681–716. https://doi.org/10.1039/C8PP90061B
Bourget CM (2008) An introduction to light-emitting diodes. Hortscience 43(7):1944–1946. https://doi.org/10.21273/HORTSCI.43.7.1944
Braga MP, Janz N (2021) Host repertoires and changing insect–plant interactions. Ecol Entomol 46:1241–1253. https://doi.org/10.1111/een.13073
Brown BA, Cloix C, Jiang GH, Kaiserli E, Herzyk P, Kliebenstein DJ, Jenkins GI (2005) A UV-B-specific signaling component orchestrates plant UV Protection. Proc Natl Acad Sci USA 102:18225–18230. https://doi.org/10.1073/pnas.0507187102
Carletti P, Masi A, Wonisch A, Grill D, Tausz M, Ferretti M (2003) Changes in antioxidant and pigment pool dimensions in UV-B irradiated maize seedlings. Environ Exp Bot 50:149–157. https://doi.org/10.1016/S0098-8472(03)00020-0
Chen Z, Gao W, Reddy KR, Chen M, Taduri S, Meyers SL, Shankle MW (2020) Ultraviolet (UV) B effects on growth and yield of three contrasting sweet potato cultivars. Photosynthetica 58:37–44. https://doi.org/10.32615/ps.2019.137
Chen Z, Ma Y, Weng Y, Yang R, Gu Z, Wang P (2019) Effects of UV-B radiation on phenolic accumulation, antioxidant activity and physiological changes in wheat (Triticum aestivum L.) Seedlings. Food Biosci 30:100409. https://doi.org/10.1016/j.fbio.2019.04.010
Christou A, Agathokleous E, Fotopoulos V (2022) Safeguarding food security: hormesis-based plant priming to the rescue. Curr Opin Environ Sci Health 28:100374. https://doi.org/10.1016/j.coesh.2022.100374
CIE (1977) Recommendations on uniform color spaces, color-difference equations, and metric color terms. Color Res Appl. https://doi.org/10.1002/j.1520-6378.1977.tb00102.x
Cloix C, Kaiserli E, Heilmann M, Baxter KJ, Brown BA, O’Hara A, Smith BO, Christie JM, Jenkins GI (2012) C-terminal region of the UV-B photoreceptor UVR8 initiates signaling through interaction with the COP1 protein. Proc Natl Acad Sci U S A 109:16366–16370. https://doi.org/10.1073/pnas.1210898109
Cuzzuol GRF, Gama VN, Zanetti LV, Werner ET, Pezzopane JEM (2020) UV-B effects on growth, photosynthesis, total antioxidant potential and cell wall components of shade-tolerant and sun-tolerant ecotypes of Paubrasilia chinate. Flora 271:151679. https://doi.org/10.1016/j.flora.2020.151679
Czégény G, Mátai A, Hideg É (2016) UV-B effects on leaves—oxidative stress and acclimation in controlled environments. Plant Sci 248:57–63. https://doi.org/10.1016/j.plantsci.2016.04.013
Diaz B, Biurrún R, Moreno A, Nebreda M, Fereres A (2006) Impact of ultraviolet-blocking plastic films on insect vectors of virus diseases infesting crisp lettuce. HortScience 41:711–716
Dolzhenko Y, Bertea CM, Occhipinti A, Bossi S, Maffei ME (2010) UV-B Modulates the interplay between terpenoids and flavonoids in peppermint (Mentha piperita L.). J Photochem Photobiol B Biol 100:67–75. https://doi.org/10.1016/j.jphotobiol.2010.05.003
Dou H, Niu G, Gu M (2019) Pre-harvest UV-B radiation and photosynthetic photon flux density interactively affect plant photosynthesis, growth, and secondary metabolites accumulation in basil (Ocimum basilicum) Plants. Agronomy 9:434. https://doi.org/10.3390/agronomy9080434
Erofeeva EA (2022) Environmental hormesis of non-specific and specific adaptive mechanisms in plants. Sci Total Environ 804:150059. https://doi.org/10.1016/j.scitotenv.2021.150059
Escobar-Bravo R, Nederpel C, Naranjo S, Kyong Kim H, Naranjo S, Rodríguez-López MJ, Chen G, Glauser G, Leiss KA, Klinkhamer PGL (2021) Ultraviolet radiation modulates both constitutive and inducible plant defenses against thrips but is dose and plant genotype dependent. J Pest Sci 94:69–81. https://doi.org/10.1007/s10340-019-01166-w
Esringu A, Aksakal O, Tabay D, Kara AA (2016) Effects of sodium nitroprusside (SNP) pretreatment on UV-B stress tolerance in lettuce (Lactuca sativa L.) seedlings. Environ Sci Pollut Res 23:589–597. https://doi.org/10.1007/s11356-015-5301-1
Faseela P, Puthur JT (2018) The imprints of the high light and UV-B stresses in Oryza Sativa L. ‘Kanchana’ seedlings are differentially modulated. J Photochem Photobiol B 178:551–559. https://doi.org/10.1016/j.jphotobiol.2017.12.009
Favory JJ, Stec A, Gruber H, Rizzini L, Oravecz A, Funk M, Albert A, Cloix C, Jenkins GI, Oakeley EJ, Seidlitz HK, Nagy F, Ulm R (2009) Interaction of COP1 and UVR8 regulates UV-B-induced photomorphogenesis and stress acclimation in Arabidopsis. EMBO J 28:591–601. https://doi.org/10.1038/emboj.2009.4
Fusco GM, Nicastro R, Rouphael Y, Carillo P (2022) The effects of the microbial biostimulants approved by EU Regulation 2019/1009 on Yield and Quality of Vegetable Crops. Foods 11:2656. https://doi.org/10.3390/foods11172656
Gange AC (2007) Insect-mycorrhizal interactions: patterns, processes and consequences. In: Ohgushi T, Craig TP, Price PW (eds) Ecological communities: plant mediation in indirect interaction webs. Cambridge University Press, New York, pp 124–144
Gange AC, West HM (1994) Interactions between arbuscular mycorrhizal fungi and foliar-feeding insects in Plantago lanceolata L. New Phytol 128:79–87. https://doi.org/10.1111/j.1469-8137.1994.tb03989.x
Gao Q, Zhang L (2008) Ultraviolet-B-induced oxidative stress and antioxidant defense system responses in ascorbate-deficient vtc1 mutants of Arabidopsis thaliana. J Plant Physiol 165:138–148. https://doi.org/10.1016/j.jplph.2007.04.002
Giovannetti M, Mosse B (1980) An evaluation of techniques for measuring vesicular arbuscular mycorrhizal infection in roots. New Phytol 84:489–500. https://doi.org/10.1111/j.1469-8137.1980.tb04556.x
Giuntini D, Lazzeri V, Calvenzani V, Dall’Asta C, Galaverna G, Tonelli C, Petroni K, Ranieri A (2008) Flavonoid profiling and biosynthetic gene expression in flesh and peel of two tomato genotypes grown under UV-B-depleted conditions during ripening. J Agric Food Chem 56:5905–5915. https://doi.org/10.1021/jf8003338
Giusti M, Wrolstad RE (2001) Characterization and measurement of anthocyanins by UV-visible spectroscopy. Curr Protoc Food Anal Chem 00:F1.2.1-F1.2.13. https://doi.org/10.1002/0471142913.faf0102s00
Goicoechea N, Garmendia I, Fabbrin EG, Bettoni MM, Palop JA, Sanmartín C (2015) Selenium fertilization and mycorrhizal technology may interfere in enhancing bioactive compounds in edible tissues of lettuces. Sci Hortic 195:163–172. https://doi.org/10.1016/j.scienta.2015.09.007
Heijde M, Ulm R (2012) UV-B photoreceptor-mediated signalling in plants. Trends Plant Sci 17:230–237. https://doi.org/10.1016/j.tplants.2012.01.007
Heinen R, Biere A, Harvey JA, Bezemer TM (2018) Effects of soil organisms on aboveground plant-insect interactions in the field: patterns, mechanisms and the role of methodology. Front Ecol Evol 6:106. https://doi.org/10.3389/fevo.2018.00106
Hideg É, Jansen MAK, Strid Å (2013) UV-B exposure, ROS, and stress: inseparable companions or loosely linked associates? Trends Plant Sci 18:107–115. https://doi.org/10.1016/j.tplants.2012.09.003
Hu J, Fang H, Wang J, Yue X, Su M, Mao Z, Zou Q, Jiang H, Guo Z, Yu L, Feng T, Lu L, Peng Z, Zhang Z, Wang N, Chen X (2020) Ultraviolet B-induced MdWRKY72 expression promotes anthocyanin synthesis in apple. Plant Sci 292:110377. https://doi.org/10.1016/j.plantsci.2019.110377
Jaiswal D, Agrawal SB (2021) Ultraviolet-B induced changes in physiology, phenylpropanoid pathway, and essential oil composition in two Curcuma species (C. caesia Roxb. and C. longa L.). Ecotoxicol Environ Saf 208:111739. https://doi.org/10.1016/j.ecoenv.2020.111739
Jaiswal D, Agrawal M, Agrawal SB (2022) Dose differentiation in elevated UV-B manifests variable response of carbon–nitrogen content with changes in secondary metabolites of Curcuma caesia Roxb. Environ Sci Pollut Res 29:72871–72885. https://doi.org/10.1007/s11356-022-20936-1
Jansen MA, Hectors K, O’Brien NM, Guisez Y, Potters G (2008) Plant stress and human health: do human consumers benefit from UV-B acclimated crops? Plant Sci 175:449–458. https://doi.org/10.1016/j.plantsci.2008.04.010
Jenkins GI (2017) Photomorphogenic responses to ultraviolet-B light. Plant Cell Environ 40:2544–2557. https://doi.org/10.1111/pce.12934
Johnson CB, Kirby J, Naxakis G, Pearson S (1999) Substantial UV-B-mediated induction of essential oils in sweet basil (Ocimum basilicum L.). Phytochemistry 51:507–510. https://doi.org/10.1016/S0031-9422(98)00767-5
Kim DO, Chun OK, Kim YJ, Moon HY, Lee CY (2003) Quantification of polyphenolics and their antioxidant capacity in fresh plums. J Agric Food Chem 51:6509–6515. https://doi.org/10.1021/jf0343074
Kliebenstein DJ, Lim JE, Landry LG, Last RL (2002) Arabidopsis UVR8 regulates ultraviolet-b signal transduction and tolerance and contains sequence similarity to human regulator of chromatin condensation 1. Plant Physiol 130:234–243. https://doi.org/10.1104/pp.005041
Koide RT, Li MG (1989) Appropriate controls for vesicular arbuscular mycorrhiza research. New Phytol 111:35–44. https://doi.org/10.1111/j.1469-8137.1989.tb04215.x
Kosobryukhov A, Khudyakova A, Kreslavski V (2020) Impact of UV radiation on photosynthetic apparatus: adaptive and damaging mechanisms. In: Hasanuzzaman M (ed) Plant ecophysiology and adaptation under climate change: mechanisms and perspectives I. Springer, Singapore, pp 555–576. https://doi.org/10.1007/978-981-15-2156-0_18
Krizek DT, Britz SJ, Mirecki RM (1998) Inhibitory effects of ambient levels of solar UV-A and UV-B radiation on growth of cv. New Red Fire Lettuce. Physiol Plant 103:1–7. https://doi.org/10.1034/j.1399-3054.1998.1030101.x
Lee M, Rivard C, Pliakoni E, Wang W, Rajashekar CB (2021) Supplemental UV-A and UV-B affect the nutritional quality of lettuce and tomato: health-promoting phytochemicals and essential nutrients. Am J Plant Sci 12:104–126. https://doi.org/10.4236/ajps.2021.121007
Li Q, Kubota C (2009) Effects of supplemental light quality on growth and phytochemicals of baby leaf lettuce. Environ Exp Bot 67:59–64. https://doi.org/10.1016/j.envexpbot.2009.06.011
Liao D, Wang S, Cui M, Liu J, Chen A, Xu G (2018) Phytohormones regulate the development of arbuscular mycorrhizal symbiosis. Int J Mol Sci 19:3146. https://doi.org/10.3390/ijms19103146
Lindroth RL, Hofmann RW, Campbell BD, McNabb WC, Hunt DY (2000) Population differences in Trifolium repens L-response to ultraviolet-B radiation: foliar chemistry and consequences for two lepidopteran herbivores. Oecologia 122:20–28. https://doi.org/10.1007/PL00008831
Masui N, Agathokleous E, Mochizuki T, Tani A, Matsuura H, Koike T (2021) Ozone disrupts the communication between plants and insects in urban and suburban areas: an updated insight focusing on plant volatiles. J for Res 32:1337–1349. https://doi.org/10.1007/s11676-020-012
Messa VR, Savioli MR (2021) Improving sustainable agriculture with arbuscular mycorrhizae. Rhizosphere 19:100412. https://doi.org/10.1016/j.rhisph.2021.100412
Mewis I, Schreiner M, Nguyen CN, Krumbein A, Ulrichs C, Lohse M, Zrenner R (2012) UV-B irradiation changes specifically the secondary metabolite profile in broccoli sprouts: induced signaling overlaps with defense response to biotic stressors. Plant Cell Physiol 53:1546–1560. https://doi.org/10.1093/pcp/pcs096
Moreira-Rodríguez M, Nair V, Benavides J, Cisneros-Zevallos L, Jacobo-Velázquez DA (2017a) UVA, UVB light doses and harvesting time differentially tailor glucosinolate and phenolic profiles in broccoli sprouts. Molecules 22:1065. https://doi.org/10.3390/molecules22071065
Moreira-Rodríguez M, Nair V, Benavides J, Cisneros-Zevallos L, Jacobo-Velázquez DA (2017b) UVA, UVB light, and methyl jasmonate, alone or combined, redirect the biosynthesis of glucosinolates, phenolics, carotenoids, and chlorophylls in broccoli sprouts. Int J Mol Sci 18:2330. https://doi.org/10.3390/ijms18112330
Mosadegh H, Trivellini A, Ferrante A, Lucchesini M, Vernieri P, Mensuali A (2018) Applications of UV-B lighting to enhance phenolic accumulation of sweet basil. Sci Hortic 229:107–116. https://doi.org/10.1016/j.scienta.2017.10.043
Moustakas M, Sperdouli I, Adamakis IDS, Moustaka J, İşgören S, Şaş B (2022) Harnessing the role of foliar applied salicylic acid in decreasing chlorophyll content to reassess photosystem II photoprotection in crop plants. Int J Mol Sci 23:7038. https://doi.org/10.3390/ijms23137038
Nascimento LBDS, Brunetti C, Agati G, Iacono CL, Detti C, Giordani E, Ferrini F, Gori A (2020) Short-term pre-harvest UV-B supplement enhances the polyphenol content and antioxidant capacity of Ocimum basilicum leaves during storage. Plants 9:797. https://doi.org/10.3390/plants9060797
Neugart S, Schreiner M (2018) UVB and UVA as eustressors in horticultural and agricultural crops. Sci Hortic 234:370–381. https://doi.org/10.1016/j.scienta.2018.02.021
Njeru EM, Avio L, Sbrana C, Turrini A, Bocci G, Bàrberi P, Giovannetti M (2014) First evidence for a major cover crop effect on arbuscular mycorrhizal fungi and organic maize growth. Agron Sustain Dev 34:841–848. https://doi.org/10.1007/s13593-013-0197-y
Pandey A, Agrawal M, Agrawal SB (2022a) Ultraviolet-B induced modifications in growth, physiology, essential oil content and composition of a medicinal herbal plant Psoralea corylifolia. Hortic Environ Biotechnol. https://doi.org/10.1007/s13580-022-00454-2
Pandey A, Agrawal M, Agrawal SB (2022b) Individual and combined effects of chromium and ultraviolet-B radiation on defense system, ultrastructural changes, and production of secondary metabolite psoralen in a medicinal plant Psoralea corylifolia L. Environ Sci Pollut Res 1–14 https://doi.org/10.1007/s11356-022-22480-4
Park JS, Choung MG, Kim JB, Hahn BS, Kim JB, Bae SC, Cho KJ (2007) Genes up-regulated during red coloration in UV-B irradiated lettuce leaves. Plant Cell Rep 26:507–516. https://doi.org/10.1007/s00299-006-0255-x
Pavela R, Benelli G (2016) Essential oils as ecofriendly biopesticides? Challenges and constraints. Trends Plant Sci 21:1000–1007. https://doi.org/10.1016/j.tplants.2016.10.005
Perez CP, Ulrichs C, Huyskens-Keil S, Schreiner M, Krumbein A, Schwarz D, Kläring HP (2008) Composition of carotenoids in tomato fruits as affected by moderate UV-B radiation before harvest. Acta Hortic 821:217–222. https://doi.org/10.17660/ActaHortic.2009.821.24
Phillips JM, Hayman DS (1970) Improved procedures for clearing roots and staining parasitic and vesicular-arbuscular mycorrhizal fungi for rapid assessment of infection. Trans Br Mycol Soc 55:158–161. https://doi.org/10.1016/S0007-1536(70)80110-3
Piccini C, Cai G, Dias MC, Romi M, Longo R, Cantini C (2020) UV-B radiation affects photosynthesis-related processes of two Italian Olea europaea (L.) varieties differently. Plants 9:1712. https://doi.org/10.3390/plants9121712
Prieto-Ruiz I, Garzo E, Moreno A, Dáder B, Medina P, Viñuela E, Fereres A (2019) Supplementary UV radiation on eggplants indirectly deters Bemisia tabaci settlement without altering the predatory orientation of their biological control agents Nesidiocoris tenuis and Sphaerophoria rueppellii. J Pest Sci 92:1057–1070. https://doi.org/10.1007/s10340-019-01103-x
Priolo A, Waghorn GC, Lanza M, Biondi L, Pennisi P (2000) Polyethylene glycol as a means for reducing the impact of condensed tannins in carob pulp: effects on lamb growth performance and meat quality. J Anim Sci 78:810–816. https://doi.org/10.2527/2000.784810x
Qaderi M, Basraon N, Chinnappa C, Reid D (2010) Combined effects of temperature, ultraviolet-b radiation, and watering regime on growth and physiological processes in canola (Brassica napus) seedlings. Int J Plant Sci 171:466–481. https://doi.org/10.1086/652389
Qi J, Zhang M, Lu C, Hettenhausen C, Tan Q, Cao G, Zhu X, Wu G, Wu J (2018) Ultraviolet-B enhances the resistance of multiple plant species to lepidopteran insect herbivory through the jasmonic acid pathway. Sci Rep 8:1–9. https://doi.org/10.1038/s41598-017-18600-7
Rai K, Agrawal SB (2021) An assessment of dose-dependent UV-B sensitivity in Eclipta alba: Biochemical traits, antioxidative properties, and wedelolactone yield. Environ Sci Pollut Res 28:45434–45449. https://doi.org/10.1007/s11356-021-13963-x
Rai K, Agrawal SB (2022) Effects of elevated ultraviolet-B on the floral and leaf characteristics of a medicinal plant Wedelia chinensis (Osbeck) Merr. along with essential oil contents. Trop Ecol 1–17. https://doi.org/10.1007/s42965-022-00270-w
Rajabbeigi E, Eichholz I, Beesk N, Ulrichs C, Kroh LW, Rohn S, Huyskens-Keil S (2013) Interaction of drought stress and UV-B radiation-impact on biomass production and flavonoid metabolism in lettuce (Lactuca sativa L.). J Appl Bot Food Qual 86. https://doi.org/10.5073/JABFQ.2013.086.026
Re R, Pellegrini N, Proteggente A, Pannala A, Yang M, Rice-Evans C (1999) Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic Biol Med 26:1231–1237. https://doi.org/10.1016/S0891-5849(98)00315-34
Rizzini L, Favory JJ, Cloix C, Faggionato D, O’Hara A, Kaiserli E, Baumeister R, Schäfer E, Nagy F, Jenkins GI, Ulm R (2011) Perception of UV-B by the Arabidopsis UVR8 protein. Science 332:103–106. https://doi.org/10.1126/science.1200660
Rodriguez C, Torre S, Solhaug KA (2014) Low levels of ultraviolet-B radiation from fluorescent tubes induce an efficient flavonoid synthesis in Lollo Rosso lettuce without negative impact on growth. Acta Agric Scand B Soil Plant Sci 64:178–184. https://doi.org/10.1080/09064710.2014.905623
Santin M, Lucini L, Castagna A, Chiodelli G, Hauser M-T, Ranieri A (2018) Post-harvest UV-B radiation modulates metabolite profile in peach fruit. Postharvest Biol Technol 139:127–134. https://doi.org/10.1016/j.postharvbio.2018.02.001
Santin M, Lucini L, Castagna A, Rocchetti G, Hauser M-T, Ranieri A (2019) Comparative “phenol-omics” and gene expression analyses in peach (Prunus persica) skin in response to different postharvest UV-B treatments. Plant Physiol Biochem 135:511–519. https://doi.org/10.1016/j.plaphy.2018.11.009
Santin M, Ranieri A, Castagna A (2021a) Anything new under the sun? An update on modulation of bioactive compounds by different wavelengths in agricultural plants. Plants 10:1485. https://doi.org/10.3390/plants10071485
Santin M, Ranieri A, Hauser M-T, Miras-Moreno B, Rocchetti G, Lucini L, Strid Å, Castagna A (2021b) The outer influences the inner: postharvest UV-B irradiation modulates peach flesh metabolome although shielded by the skin. Food Chem 338:127782. https://doi.org/10.1016/j.foodchem.2020.127782
Santin M, Sciampagna MC, Mannucci A, Puccinelli M, Angelini LG, Tavarini S, Castagna A (2022) Supplemental UV-B exposure influences the biomass and the content of bioactive compounds in Linum usitatissimum L. sprouts and microgreens. Hortic 8:213. https://doi.org/10.3390/horticulturae8030213
Schreiner M, Mewis I, Huyskens-Keil S, Jansen MAK, Zrenner R, Winkler JB, O’Brien N, Krumbein A (2012) UV-B-induced secondary plant metabolites – potential benefits for plant and human health. Crit Rev Plant Sci 31:229–240. https://doi.org/10.1080/07352689.2012.664979
Sharma E, Anand G, Kapoor R (2017) Terpenoids in plant and arbuscular mycorrhiza-reinforced defence against herbivorous insects. Ann Bot 119:791–801. https://doi.org/10.1093/aob/mcw263
Sharma G, Malthankar PA, Mathur V (2021) Insect–plant interactions: a multilayered relationship. Ann Entomol Soc Am 114:1–16. https://doi.org/10.1093/aesa/saaa032
Sheng K, Zheng H, Shui S, Yan L, Liu C, Zheng L (2018) Comparison of postharvest UV-B and UV-C treatments on table grape: changes in phenolic compounds and their transcription of biosynthetic genes during storage. Postharvest Biol Technol 138:74–81. https://doi.org/10.1016/j.postharvbio.2018.01.002
Singh S, Kumari R, Agrawal M, Agrawal SB (2011) Modification in growth, biomass and yield of radish under supplemental UV-B at different NPK levels. Ecotoxicol Environ Saf 74:897–903. https://doi.org/10.1016/j.ecoenv.2010.12.011
Smith SE, Read D (2008) Mycorrhizal symbiosis, 3rd edn. Academic Press, San Diego, USA
Sorour MA, Khamiss OA, El-Wahab AS, El-Sheikh MA, Abulela S (2011) An economically modified semi-synthetic diet for mass rearing the Egyptian cotton leaf worm Spodoptera littolaris. Ac J Entomol 4:118–123
Stracke R, Favory JJ, Gruber H, Bartelniewoehner L, Bartels S, Binkert M, Ulm R (2010) The Arabidopsis bZIP transcription factor HY5 regulates expression of the PFG1/MYB12 gene in response to light and ultraviolet-B radiation. Plant Cell Environ 33:88–103. https://doi.org/10.1111/j.1365-3040.2009.02061.x
Takshak S, Agrawal SB (2019) Defense potential of secondary metabolites in medicinal plants under UV-B stress. J Photochem Photobiol b, Biol 193:51–88. https://doi.org/10.1016/j.jphotobiol.2019.02.002
Tavarini S, Castagna A, Conte G, Foschi L, Sanmartin C, Incrocci L, Ranieri A, Serra A, Angelini LG (2019) Evaluation of chemical composition of two linseed varieties as sources of health-beneficial substances. Molecules 24:3729. https://doi.org/10.3390/molecules24203729
Teramura AE, Ziska AH, Sztein LH (1991) Changes in growth and photosynthetic capacity of rice with increased UV-B radiation. Physiol Plant 83:373–380. https://doi.org/10.1111/j.1399-3054.1991.tb00108.x
Tevini M, Iwanzik W, Thoma U (1981) Some effects of enhanced UV-B irradiation on the growth and composition of plants. Planta 153:388–394. https://doi.org/10.1007/BF00384258
Tsormpatsidis E, Henbest RGC, Davis FJ, Battey NH, Hadley P, Wagstaffe A (2008) UV irradiance as a major influence on growth, development and secondary products of commercial importance in Lollo Rosso lettuce ‘Revolution’ grown under polyethylene films. Environ Exp Bot 63:232–239. https://doi.org/10.1016/j.envexpbot.2007.12.002
Ubi BE, Honda C, Bessho H, Kondo S, Wada M, Kobayashi S, Moriguchi T (2006) Expression analysis of anthocyanin biosynthetic genes in apple skin: effect of UV-B and temperature. Plant Sci 170:571–578. https://doi.org/10.1016/j.plantsci.2005.10.009
Vandenbussche F, Yu N, Li W, Vanhaelewyn L, Hamshou M, Van Der Straeten D, Smagghe G (2018) An ultraviolet B condition that affects growth and defense in Arabidopsis. Plant Sci 268:54–63. https://doi.org/10.1016/j.plantsci.2017.12.005
Vangelisti A, Natali L, Bernardi R, Sbrana C, Turrini A, Hassani-Pak K, Hughes D, Cavallini A, Giovannetti M, Giordani T (2018) Transcriptome changes induced by arbuscular mycorrhizal fungi in sunflower (Helianthus annuus L.) roots. Sci Rep 8:4. https://doi.org/10.1038/s41598-017-18445-0
Vannette RL, Hunter MD (2011) Plant defence theory re-examined: nonlinear expectations based on the costs and benefits of resource mutualisms. J Ecol 99:66–76. https://doi.org/10.1111/j.1365-2745.2010.01755.x
Vicari M, Hatcher PE, Ayres PG (2002) Combined effect of foliar and mycorrhizal endophytes on an insect herbivore. Ecology 83:2452–2464. https://doi.org/10.1890/0012-9658(2002)083[2452:CEOFAM]2.0.CO;2
Volkova PY, Bondarenko EV, Kazakova EA (2022) Radiation hormesis in plants. Curr Opin Toxicol 30:100334. https://doi.org/10.1016/j.cotox.2022.02.007
Wang H, Gui M, Tian X, Xin X, Wang T, Li J (2017) Effects of UV-B on vitamin C, phenolics, flavonoids and their related enzyme activities in mung bean sprouts (Vigna radiata). Int J Food Sci Technol 52:827–833. https://doi.org/10.1111/ijfs.13345
Wargent JJ, Jordan BR (2013) From ozone depletion to agriculture: understanding the role of UV radiation in sustainable crop production. New Phytol 197:1058–1076. https://doi.org/10.1111/nph.12132
Wellburn AR (1994) The spectral determination of chlorophylls a and b, as well as total carotenoids, using various solvents with spectrophotometers of different resolutions. J Plant Physiol 144:307–313. https://doi.org/10.1016/S0176-1617(11)81192-2
Yadav A, Singh D, Lingwan M, Yadukrishnan P, Masakapalli SK, Datta S (2020) Light signaling and UV-B-mediated plant growth regulation. J Integr Plant Biol 62:1270–1292. https://doi.org/10.1111/jipb.12932
Yu L, Zhang W, Geng Y, Liu K, Shao X (2022) Increases plant nutrient uptake and improves defenses against insects. Front Ecol Evol 10. https://doi.org/10.3389/fevo.2022.833389
Zeni V, Grassi A, Santin M, Ricciardi R, Pieracci Y, Flamini G, Di Giovanni F, Marmugi M, Agnolucci M, Avio L, Turrini A, Giovannetti M, Castiglione MR, Ranieri A, Canale A, Lucchi A, Agathokleous E, Benelli G (2023) Leaf UV-B irradiation and mycorrhizal symbionts affect lettuce VOC emissions and defence mechanisms, but not aphid feeding preferences. Insects 14(1):20. https://doi.org/10.3390/insects14010020
Zhang L, Gong F, Song Y, Liu K, Wan Y (2019) De novo transcriptome analysis of lettuce (Lactuca sativa L.) and the identification of structural genes involved in anthocyanin biosynthesis in response to UV-B radiation. Acta Physiol Plant 41:1–16. https://doi.org/10.1007/s11738-019-2941-7
Zhu B, Gao T, Zhang D, Ding K, Li C, Ma F (2022) Functions of arbuscular mycorrhizal fungi in horticultural crops. Sci Hort 303:111219. https://doi.org/10.1016/j.scienta.2022.111219
Funding
Open access funding provided by Università di Pisa within the CRUI-CARE Agreement. This study is funded by the University of Pisa PRA_2020_19, “Boosting plant tolerance to insect pests through UV light exposure and mycorrhizal symbionts.”
Author information
Authors and Affiliations
Contributions
Marco Santin: conceptualization, data curation, investigation, software, and writing — original draft. Valeria Zeni: conceptualization, data curation, investigation, software, and writing — original draft. Arianna Grassi: formal analysis, investigation, methodology, visualization, and writing — review and editing. Renato Ricciardi: formal analysis, investigation, methodology, visualization, and writing — review and editing. Ylenia Pieracci: formal analysis, methodology, visualization, and writing — review and editing. Filippo Di Giovanni: formal analysis, investigation, methodology, visualization, and writing — review and editing. Sofia Panzani: formal analysis, investigation, methodology, visualization, and writing — review and editing. Christian Frasconi: formal analysis, methodology, visualization, and writing — review and editing. Monica Agnolucci: conceptualization, data curation, resources, supervision, and writing — original draft. Luciano Avio: conceptualization, data curation, resources, supervision, and writing — original draft. Alessandra Turrini: conceptualization, data curation, resources, supervision, writing — original draft. Manuela Giovannetti: conceptualization, data curation, resources, supervision, and writing — review and editing. Monica Ruffini Castiglione: data curation, resources, supervision, writing — original draft, and writing — review and editing. Annamaria Ranieri: Conceptualization, Data curation, Resources, Supervision, and writing — review and editing. Angelo Canale: formal analysis, methodology, visualization, and writing — review and editing. Andrea Lucchi: formal analysis, methodology, visualization, and writing — review and editing. Evgenios Agathokleous: formal analysis, methodology, supervision, visualization, and writing — review and editing. Giovanni Benelli: conceptualization, data curation, formal analysis, funding acquisition, investigation, project administration, resources, supervision, writing — original draft. All the authors approved the final version of the manuscript.
Corresponding author
Ethics declarations
Ethical approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Responsible Editor: Philippe Garrigues
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Santin, M., Zeni, V., Grassi, A. et al. Do changes in Lactuca sativa metabolic performance, induced by mycorrhizal symbionts and leaf UV-B irradiation, play a role towards tolerance to a polyphagous insect pest?. Environ Sci Pollut Res 30, 56207–56223 (2023). https://doi.org/10.1007/s11356-023-26218-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-023-26218-8