Abstract
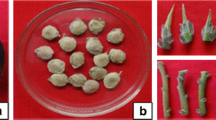
Tuberization in many terrestrial orchids represents the most important physiological process for reproduction and survival. An in vitro controlled and reproducible tuberization and plant regeneration system was designed for Habenaria bractescens. Multinodal segments were incubated on Murashige and Skoog (MS) medium supplemented with different concentrations of N6-benzylaminopurine (BAP) and sucrose. After 45 days, the explants developed root tubers in vitro in 8 of the 12 media assayed. MS medium with 87.6 mM sucrose plus 4.4 μM BAP was one of the most effective for stimulating root tubers. Plants derived from in vitro root tubers were successfully transferred to the greenhouse without any acclimatization. The morphology and anatomy of the regenerated underground organs were examined to find identifying and distinguishing features. The protocol to regenerate root tubers of H. bractescens will be useful to study the basic aspects and control of tuberization and to carry out restoration programs.




Similar content being viewed by others
References
Agarwal PK, Ranu RS (2000) Regeneration of plantlets from leaf and petiole explants of Pelargonium × hortorum. In Vitro Cell Dev Biol Plant 36:392–397. doi:10.1007/s11627-000-0070-y
Barroso J, Fevereiro P, Oliveira MM, Pais MSS (1990) In vitro seed germination, differentiation and production of minitubers from Ophrys lutea Cav., Ophrys fusca Link and Ophrys speculum Link. Sci Hortic (Amsterdam) 42:329–337. doi:10.1016/0304-4238(90)90056-K
Bell AD (1993) Plant form: an illustrated guide to flowering plant morphology. Oxford University Press, Oxford
Chang C, Chang WC (1998) Plant regeneration from callus culture of Cymbidium ensifolium var misericors. Plant Cell Rep 17:251–255. doi:10.1007/s002990050387
Correa MN (1996) Orchidaceae. In: Zuloaga F, Morrone O (eds) Catálogo de plantas vasculares de la República Argentina I, Monogr Syst Bot Missouri Bot Gard, vol 60. Missouri Botanical Garden, Missouri, USA, pp 242–271
Debeljak N, Regvar M, Dixon K, Sivasithamparam K (2002) Induction of tuberization in vitro with jasmonic acid and sucrose in an Australian terrestrial orchid, Pterostylis sanguinea. Plant Growth Regul 36:253–260. doi:10.1023/A:1016570319387
Dressler R (1993) Phylogeny and classification of the orchid family. Dioscorides Press, Hong Kong
Fernie AR, Willmitzer L (2001) Update on tuber formation, dormancy and sprouting: molecular and biochemical triggers of potato tuber development. Plant Physiol 127:1459–1465. doi:10.1104/pp.010764
Franklin G, Dias A (2006) Organogenesis and embryogenesis in several Hypericum perforatum genotypes. In Vitro Cell Dev Biol Plant 42:324–330. doi:10.1079/IVP2006787
Ghosh S, Ghosh B, Jha S (2007) In vitro tuberization of Gloriosa superba L. on basal medium. Sci Hortic (Amsterdam) 114:220–223. doi:10.1016/j.scienta.2007.06.008
Goleniowski M, Del Longo O, De Forchetti SM, Argüello JA (2001) Relationships between peroxidases and in vitro bulbification in garlic (Allium sativum L.). In Vitro Cell Dev Biol Plant 37:683–686. doi:10.1007/s11627-001-0119-6
Gonzalez AM, Cristóbal CL (1997) Anatomía y ontogenia de semillas de Helicteres Lhotzkyana (Sterculiaceae). Bonplandia 9:287–294
Hoehne FC (1940) Flora Brasilica, Fasc 12. Secretaria da Agricultura. Indústria e Comércio de São Paulo, São Paulo
Hollick PS, Senaratna T, McComb JA, Bunn E, Dixon KW (2001) Response to paclobutrazol of symbiotic mycorrhizal fungi and dropper (tuber stalk) formation of host orchid seedlings. Plant Growth Regul 36:31–39. doi:10.1023/A:1014799008459
Hussey G, Stacey NJ (1984) Factors affecting the formation of in vitro tubers of potato (Solanum tuberosum L.). Ann Bot (Lond) 53:565–578
Ilczuk A, Winkelmann T, Richartz S, Witomska M, Serek M (2005) In vitro propagation of Hippeastrum × chmielii Chm.—influence of furprimidol and the culture in solid or liquid medium and in temporary immersion systems. Plant Cell Tissue Organ Cult 83:339–346. doi:10.1007/s11240-005-8812-5
InfoStat versión 1.1 (2002) Manual del Usuario. Grupo InfoStat, FCA, Universidad Nacional de Córdoba, 1st edn. Editorial Brujas, Córdoba, Argentina, pp 1–276
Jackson SD (1999) Multiple signaling pathways control tuber induction in potato. Plant Physiol 119:1–8. doi:10.1104/pp.119.1.1
Johansen DA (1940) Plant microtechnique. McGraw-Hill, New York
Johnson E (2001) Las orquídeas del Parque Nacional Iguazú. LOLA, Buenos Aires, Argentina
Kumazawa M (1958) The sinker of Platanthera and Perularia—its morphology and development. Phytomorphology 8:137–145
Loizaga de Castro N, Arbo MM (2005) Anatomía de tallo y órganos subterráneos en Habenaria bractescens Lindl. (Orchidaceae). Proceedings X Scientific and Technological Meeting of the UNNE, Biology area, Corrientes, Argentina, p 12
Luque R, Sousa HC, Kraus JE (1996) Métodos de coloração de Roeser (1972)-modificado- e Kropp (1972) visando a subtituição do azul de astra por azul de alcião 8 GS ou 8 GX. Acta Bot Bras 10:199–212
Mabberley DJ (1993) The plant book. Cambridge University Press, Cambridge
Medina RD, Faloci MM, Gonzalez AM, Mroginski LA (2007) In vitro cultured primary roots derived from stem segments of cassava (Manihot esculenta) can behave like storage organs. Ann Bot (Lond) 99:409–423. doi:10.1093/aob/mcl272
Melis RJM, van Staden J (1984) Tuberization and hormones. Z Pflanzenphysiol 113:271–283
Murashige T, Skoog F (1962) A revised medium for rapid growth and bioassay with tobacco tissue cultures. Physiol Plant 15:473–497. doi:10.1111/j.1399-3054.1962.tb08052.x
Ndoumou DO, Tsala GN, Kanmegne G, Balangé AP (1995) In vitro induction of multiple shoots, plant regeneration and tuberization from shoot tips of cocoyam. C R Acad Sci París, Sciences de la vie 318:773–778
Omokolo ND, Boudjeko T, Tsafack Takadong JJ (2003) In vitro tuberization of Xanthosoma sagittifolium L. Schott: effects of phytohormones, sucrose, nitrogen and photoperiod. Sci Hortic (Amsterdam) 98:337–345. doi:10.1016/S0304-4238(03)00066-9
Oyono PO, Kevers EC, Dommes EJ (2007) Axillary proliferation and tuberisation of Dioscorea cayenensis—D. rotundata complex. Plant Cell Tissue Organ Cult 91:107–114. doi:10.1007/s11240-007-9238-z
Pedroso MC, Pais MS (1992) Minituber production from immature seed suspension culture of Orchis papilionacea. In Vitro Cell Dev Biol Plant 28:183–186. doi:10.1007/BF02823314
Piao XC, Chakrabarty D, Hahn EJ, Paek KY (2003) A simple method for mass production of potato microtubers using a bioreactor system. Curr Sci 84:1129–1132
Poornima G, Ravishankar Rai V (2007) In vitro propagation of wild yams, Dioscorea oppositifolia L. and Dioscorea pentaphylla L. Afr J Biotechnol 20:2348–2352
Prakash MG, Gurumurthi K (2004) Effect of age of explants on somatic embryogenesis and plant regeneration in Eucalyptus tereticornis Sm. Plant Cell Biotechnol Mol Biol 5:39–44
Pridgeon AM, Chase MW (1995) Subterranean axes in Tribe Diurideae (Orchidaceae): morphology, anatomy, and systematic significance. Am J Bot 82:1473–1491. doi:10.2307/2446176
Pridgeon AM, Cribb PJ, Chase MW, Rasmussen FN (1999) Genera Orchidacearum. Vol 1. General Introduction. Apostasioideae. Cypripedioideae. Oxford University Press, Oxford
Pridgeon AM, Cribb PJ, Chase MW, Rasmussen FN (2001) Genera Orchidacearum. Vol 2. Orchidoideae (Part One). Oxford University Press, Oxford
Pridgeon AM, Cribb PJ, Chase MW, Rasmussen FN (2003) Genera Orchidacearum. Vol 3. Orchidoideae (Part Two). Vanilloideae. Oxford University Press, Oxford
Rego-Oliveira LV, Faria RT, Batista Fonseca IC, Saconato Ch (2003) Influência da fonte e concentração de carboidrato no crescimento vegetativo e enraizamento in vitro de Oncidium varicosum Lindl. (Orchidaceae). Semina. Cienc Agrarias 24:265–272
Roy J, Banerjee N (2002) Rhizome and shoot development during in vitro propagation of Geodorum densiflorum (Lam.) Schltr. Sci Hortic (Amsterdam) 94:181–192. doi:10.1016/S0304-4238(01)00373-9
Sarkar D (2008) The signal transduction pathways controlling in planta tuberization in potato: an emerging synthesis. Plant Cell Rep 27:1–8. doi:10.1007/s00299-007-0457-x
Sengupta J, Mitra GC, Sharma AK (1984) Organogenesis and tuberization in cultures of Dioscorea floribunda. Plant Cell Tissue Organ Cult 3:325–331. doi:10.1007/BF00043084
Shimada T, Otani M, Morii M, Godo T (2001) In vitro propagation and recovery of a habitat of Habenaria radiata (Orchidaceae). In: Sorvari S, Karhu S, Kanervo E, Pihakaski S (eds) Proc. IV IS on In vitro Cult. & Hort Breeding, Acta Hort 560, ISHS, pp 481–483
Shirgurkar MV, John CK, Nadgauda RS (2001) Factors affecting in vitro microrhizome production in turmeric. Plant Cell Tissue Organ Cult 64:5–11. doi:10.1023/A:1010645624618
Stern WL (1997) Vegetative anatomy of subtribe Habenariinae (Orchidaceae). Bot J Linn Soc 125:211–227
Stewart SL, Kane ME (2006) Asymbiotic seed germination and in vitro seedling development of Habenaria macroceratitis (Orchidaceae), a rare Florida terrestrial orchid. Plant Cell Tissue Organ Cult 86:147–158. doi:10.1007/s11240-006-9098-y
Struik PC, Vreugdenhil D, van Eck HJ, Bachem CW, Visser RGF (1999) Physiological and genetic control of tuber formation. Potato Res 42:313–331. doi:10.1007/BF02357860
Tressens SG, Arbo MM (2002) Introduction. In: Arbo MM, Tressens SG (eds) Flora del Iberá. EUDENE, Corrientes, Argentina, pp 3–7
Vreugdenhil D, Sergeeva LI (1999) Gibberellins and tuberization in potato. Potato Res 42:471–481. doi:10.1007/BF02358163
Weston PH, Perkins AJ, Entwisle TJ (2005) More than symbioses: orchid ecology, with examples from the Sydney Region. Cunninghamia 9:1–15
Wotavová-Novotná K, Vejsadová H, Kindlmann P (2007) Effects of sugars and growth regulators on in vitro growth of Dactylorhiza species. Biol Plant 51:198–200. doi:10.1007/s10535-007-0040-x
Xu X, Vreugdenhil D, van Lammeren AAM (1998) Cell division and cell enlargement during potato tuber formation. J Exp Bot 49:573–582. doi:10.1093/jexbot/49.320.573
Yamamoto T, Saito Y, Yamamoto C, Kinjo N (2001) Restoration of Habenaria radiata propagated from aseptic seeding to the habitat. Proceedings VII Asia Pacific Orchid Conference, pp 48–50
Zettler LW (1997) Terrestrial orchid conservation by symbiotic seed germination: techniques and perspectives. Selbyana 18:188–194
Zhou SP, He YK, Li SJ (1999) Induction and characterization of in vitro corms of diploid-taro. Plant Cell Tissue Organ Cult 57:173–178. doi:10.1023/A:1006335813056
Acknowledgments
The authors are grateful to the FPLA’S (Fondo Para Las Américas), FORA (Federación Civil de Orquideófilos de la República Argentina), CONICET and SGCyT (Secretaría General de Ciencia y Técnica, UNNE) for the financial support. The authors wish to express their gratitude to Beth Hazen, Ph.D. and Ernestina Galdeano, Ph.D., for their valuable comments on the manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Medina, R.D., Flachsland, E.A., Gonzalez, A.M. et al. In vitro tuberization and plant regeneration from multinodal segment culture of Habenaria bractescens Lindl., an Argentinean wetland orchid. Plant Cell Tiss Organ Cult 97, 91–101 (2009). https://doi.org/10.1007/s11240-009-9505-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11240-009-9505-2