Abstract
Calluna vulgaris (L.) Hull is primarily found on acid soils and is generally classified as a calcifuge species. Therefore, its occasional growth in semi-arid grassland on shallow calcareous soils gave rise to the question as to whether special soil conditions, deviating from the typical conditions in calcareous soils, enable this unusual occurrence. In an attempt to answer this question, we analysed selected soil factors, comparing plots where C. vulgaris was growing besides calcicole species (=CF plots) with neighbouring plots where only calcicole species were present (=CC plots). Main emphasis was placed on Fe and P availability because results from growth experiments indicate that the availability of either Fe or P to calcifuges causes calcifuge species to fail on calcareous soils. The results of our investigations do not support the hypothesis that the occurrence of C. vulgaris in semi-arid calcareous grasslands depends on higher Fe and/or P availability. Rather, its growth on carbonate-buffered soils shows that this species is not really calcifuge. Since the CF plots differ from the CC plots either by a lower inclination or by a more northerly exposition, we assume that the primary establishment and this species’ distribution pattern in the investigated semi-arid grassland are not dependent on soil chemical factors, but are governed by topography and its consequences for soil humidity and drought stress.
Similar content being viewed by others
Introduction
C. vulgaris (L.) Hull is normally regarded as a calcifuge species (Gimingham 1960; Ellenberg et al. 1992) because it is a frequent component of various vegetation types on acid to very acid soils. However, the species occurs occasionally even in semi-arid grasslands on shallow calcareous soils, where it grows in mixtures with calcicole plant species (Grubb et al. 1969). Under conditions where calcareous soils are superficially acidified or where layers of non-calcareous, acidic material, as e.g. loess or humus, overlie the calcareous soil material, the occurrence of C. vulgaris is still in accordance with its calcifuge classification. However, there are also sites where this and other species, regarded as more or less calcifuge, are present on calcareous soils that lack such acidic layers and where the root system of these plants penetrate almost down to the bedrock. Such cases raise the question as to whether special soil conditions, deviating from normal conditions in calcareous soils, allow the growth of these species or whether they are not calcifuge in a strict sense.
Examples of sites where C. vulgaris and some moderately calcifuge species grow in mixtures with calcicole species are to be found in semi-arid calcareous grasslands in southern Lower Saxony and northern Hesse in the centre of Germany. In these cases the calcifuge species are not equally distributed over the total area but occur in scattered patches. This offers a possibility to answer the question, raised above, by comparing soils at plots where C. vulgaris is mixed with calcicole and indifferent species with soils at plots where exclusively calcicole and indifferent species are growing. If soil factors that differ between these locations could be identified, this would contribute to an answer to the more general question of why calcifuge species normally avoid calcareous soils.
Above all, two factors deserve particular attention in this context:
-
(a)
Soil iron solubility is, as a general rule, minimal between pH 7.4 and pH 8.5, i. e., within the pH range of CaCO3 buffering at typical partial pressures of CO2 in the soil solution (Lindsay 1979). In addition, uptake and metabolism of Fe are disturbed by high pH (Susin et al. 1996; Kosegarten et al. 1999) and high HCO3 − concentrations (Lee and Woolhouse 1969a, b; Mengel 1994). As both factors are usually high in calcareous soils, plants lacking the ability to solubilize, to take up, or to metabolise Fe under these conditions can be expected to fail. For a long time, this has been assumed to be decisive for the exclusion of calcifuge species from calcareous soils (Grime 1963). In fact, a weaker ability to acquire and to utilise Fe has been observed in several calcifuge plants in comparison to calcicole ones (Snaydon and Bradshaw 1962; Grime and Hodgson 1969; Gries and Runge 1995; Zohlen and Tyler 2000).
-
(b)
Plant phosphate availability is restricted in calcareous soils because high Ca2+ concentrations and high pH lead to precipitation of Ca phosphates and, ultimately, to the formation of apatites (Lindsay et al. 1989). P deficiency has been found to prevent several calcifuge species from growing on calcareous soils (Tyler 1992, 1994; Zohlen and Tyler 2004), and has been explained by assuming a weaker ability of these species to solubilize phosphate compared to calcicole species (Ström et al. 1994; Tyler and Ström 1995; Ström 1997).
Proceeding from these considerations, we hypothesised that (a) Fe availability or (b) P availability is favoured by special soil conditions in patches where C. vulgaris grows together with calcicole species compared to patches where only calcicole species are found. To test this hypothesis, we determined the soil contents of different fractions of Fe and P together with other factors that influence the availability of these elements, applying both standard and novel extraction methods.
Material and methods
Study sites
Detailed investigations were performed for two semi-arid calcareous grasslands (study sites I and II) situated on a Triassic limestone plateau north of Kassel, Germany. In addition to these main sites investigations were conducted for comparable semi-arid calcareous grasslands at four supplementary sites. The study sites III and IV are adjacent to the main sites, whereas the sites V and VI are located at the south-western foothills of the Harz mountains (Table 1).
Parent material of soil formation at study sites I–IV is limestone originating from Lower Muschelkalk, at sites V–VI carbonate-rich sediments of the Stassfurt series in the Upper Permian Zechstein formation. According to the WRB classification, soil types are either shallow rendzic leptosol or mollic leptosols with a somewhat deeper solum (FAO 2006; Table 3). These soils are representative for calcareous grasslands in Central Western Europe (Niklaus and Falloon 2006). Soil organic carbon contents (C o) mostly range between 2.0% and 6.0% (m/mDW), corresponding to carbon contents widespread in calcareous soils under temperate climatic conditions (e.g. Misra and Tyler 1999; Niklaus 1998; Dušek 1995).
Plots of 100 m2 (10 × 10 m) were installed both in patches with and without C. vulgaris at the main sites I and II (in the following, plots with C. vulgaris are denoted CF plots, the others CC plots, the soils analogously as CF soils and CC soils). The plots areas were divided into 16 subplots (2.5 × 2.5 m). The vegetation was recorded in eight alternating subplots. At the supplementary sites III–VI plants were recorded in a 2.5 × 2.5 m plot within each vegetation type. The nomenclature follows Wisskirchen and Häupler (1998) for phanerogams and Koperski et al. (2000) for bryophytes. The complete lists of the species and the results of detailed plant sociological surveys of the study sites are given in Fühner (2005).
Soil sampling and preparation of soil material
Soil depths were determined by inserting a graduated metal rod in regular intervals (145 insertions per plot at the main sites, 10 insertions per plot at the supplementary sites). Soil samples were taken from the upper 10 cm of the Ah-horizon using a brass cylinder (height 118 mm, inner diameter 52 mm). Above-ground plant material and the organic layer were removed, the upper 200 ml of the soil columns filled into PE bags, transported to the laboratory in a cool box and stored at 4°C until preparation for soil analyses.
Soil material was sampled at the main sites, I and II, on seven dates distributed across the year. On each date samples were taken from eight alternating subplots within each vegetation type. At the supplementary sites III–VI, soil sampling was done only once. Four samples were taken there at random within the plots where the vegetation analyses had been made.
Soil material was weighed and sieved (2 mm mesh) within 24 h after sampling. The coarse material was washed, divided into skeleton material and roots, dried and weighed. Fine soil homogenates were used for further procedures either field-moist or air-dried.
Determination of water content and weight density of fine soil
The water content of the field-moist fine soil homogenate was determined by drying subsamples of 10 g at 106°C and cooling them in a desiccator before weighing. Based on the gravimetric water content of the fine soil homogenate and the specific density of the skeleton material (2.65 g/ml) the volumetric water content Θ and the weight density ρ of the fine soil fraction of the intact soil columns were calculated.
Preparation of equilibrium soil solutions (ESS)
ESS were prepared in accordance with Ludwig et al. (1999). After sieving, H2O demin. was added to 25 g field-moist fine soil in a 100 ml Erlenmeyer flask to such an amount that under consideration of the actual water content a soil/water ratio of 1:2 was attained. The flask was sealed and shaken horizontally for 24 h. Subsequently, the soil suspension was centrifuged twice (2 min, g max = 100,000×g, T = 4°C), the supernatant filtered through cellulose acetate filter (Sartorius, 0.45 μm), and the filtrate stored at 4°C until analysis.
Determination of effective cation exchange capacities (CECeff)
CECeff was determined following Falkengren-Grerup et al. (1995). A suspension of 5.0 g air-dry fine soil in 50 ml 0.1 M BaCl2 was shaken for 2 h in a 100 ml Erlenmeyer flask. Subsequently, the suspension was centrifuged (10 min, 1,500×g) and the supernatant filtered (Schleicher & Schuell 5893). The pH of the filtrate was measured electrometrically, the cation concentrations were determined by atomic absorption spectrophotometry (AAS).
Non-sequential fractionation of iron
To evaluate the plant Fe availability at the study sites, field moist fine soil was extracted with H2O demin. and with solutions of Ferrozine, DTPA, NTA, and Oxalate.
Ferrozine (PDTS, 3-(2-Pyridyl)-5,6-diphenyl-1,2,4-triazin-4′,4″ disulfonic acid monosodium salt) forms a stable 3 : 1 complex with Fe2+ that can be used for colorimetric quantification of ferrous iron. In order to minimise oxidative Fe precipitation during the extraction 20 g field-moist fine soil were mixed immediately after sieving the bulk soil with 60 ml of 500 μM Ferrozine solution. After shaking the suspension horizontally for 24 h and ultracentrifugation (30 min, gmax = 100 000 ∙ g, T = 4°C) ferrous iron in the supernatant was determined photometrically.
DTPA (diethylenetriaminepentaacetic acid) is widely used for the quantification of “plant-available” Fe, but also of Cu, Mn and Zn in calcareous soils. Solutions of DTPA (5 mM), buffered with triethanolamine (0.1 M) to pH 7.3, extract mainly an exchangeable fraction of the metals (Lindsay and Norvell 1978). In order to prevent the dissolution of CaCO3 from the soil material 0.01 M CaCl2 were added to the extractant. Suspensions of 25 g field-moist fine soil and 50 ml extractant solution were shaken for 4 h (deviating from the original protocol ) before being filtered through filter paper (Schleicher & Schuell 5893).
NTA (nitrilotriacetate) was used to determine organically bound Fe. Following Yuan et al. (1993), 50 ml of 0.1 M NTA solution (pH 8.3) were added to 1.0 g field-moist fine soil, and the suspension was shaken for 24 h before filtering through filter paper (Schleicher & Schuell 5893). A preceding treatment of the soil homogenate with 0.01 M HCl, included in the original protocol, was omitted because it reduced the extracted Fe amount from air-dry fine soil homogenates in preliminary experiments for 2.6% only.
Oxalate was used to quantify amorphous, non-crystalline Fe-(oxy/hydr)oxides. Being a strong ligand, oxalate also extracts Fe from organic compounds (Campbell and Schwertmann 1984; Borggaard 1988). For the extraction 50 ml ammonium oxalate/oxalic acid (0.2 M, pH 3.25) were added to 5.0 g field-moist fine soil. The suspension was shaken for 4 h in darkness and finally filtered (Schleicher & Schuell 5893).
Sequential P fractionation
The plant P availability at the study sites was evaluated according to the sequential fractionation scheme of Tiessen and Moir (1993) with H2O in the presence of an anion exchange resin, NaHCO3, NaOH, and HCl. Residual P fractions were not extracted because their importance in the P supply to plants is insignificant. In each step 30 ml of the extractant were added to 0.5 g air-dried, ground soil material in polycarbonate tubes, and the suspensions were shaken vertically for 16 h. Solution and solid matter were separated by ultracentrifugation (30 min, g max = 25,000×g, T = 4°C). Remaining particles were removed by filtering the supernatant through a cellulose acetate filter (0.45 μm, Sartorius) before photometric P analyses.
To determine the water soluble and easily exchangeable Presin a basic anion exchanger (Dowex® 1 × 8, 20–50 mesh) was used. A polyethylene net containing 4 cm3 of the exchanger was added to 500 mg of the soil material and 30 ml H2O demin. After shaking, the attached soil material was rinsed off the net twice with 10 ml H2O demin. P was desorbed by shaking the net for 30 min in 25 ml 0.5 M HCl. Before the next step, extraction and rinsing solutions were combined, and the solid matter was separated from the combined solutions by centrifugation.
NaHCO3 was used to determine exchangeable P as well as inorganic and organic P that is weakly bound to soil aggregates (Olsen-P). Because of the high respiration rates in the rhizosphere, which promote the dissolution of CaCO3 as well as the desorption of P by HCO3 −, this method characterises a plant available P fraction especially in calcareous soils. As the proportions of the dissociation species in the carbonate system are pH-dependent and to ensure a concentration of 0.5 M HCO3 −, the NaHCO3 solution was brought to pH 8.5 with NaOH.
Hydroxide ions dissolve P that is fixed to Fe and Al (hydr)oxides as well as P from base-soluble organic substances. For the extraction of this fraction 0.1 M NaOH was used.
To extract the P fraction that is bound as apatite, the soil material was finally shaken with 1.0 M HCl.
Analytical procedures
Actual acidities (pHH2O) and potential acidities (pHKCl) were determined electrometrically in 10 g of field-moist fine soil equilibrated with 20 ml of H2O demin. and 1.0 M KCl solutions, respectively. Additionally, pH was measured in the ESS and in the solutions of the CEC determination.
The carbonate content of the fine soil was measured volumetrically with a Scheibler apparatus, based on the release of CO2 when HCl is added to the soil (Loeppert and Suarez 1996).
Total contents of C and N were measured with a C–N analyser (Carlo Erba NA 1500) in 10 mg air-dried and ground fine soil. The contents of organic C were calculated by subtracting the inorganic C content from total C.
DOC concentrations in the ESS of CF soils and CC soils from the main sites were analysed for one sampling date using a TOC analyser (Shimadzu TOC-5000/5050).
The P and NO3 − concentrations of the ESS were measured by ion chromatography, using a combined Gynkotek/Waters HPLC system controlled by the Dionex chromatography software Chromeleon (version 4.20). Because of the high cation contents of the ESS a preparative cation exchange had to be performed before anion analysis. About 1 ml ESS was poured into a tube filled with a cation exchanger (Amberlite CG-120II, 200–400 mesh). The first few drops were discarded, and the rest was filled directly into an autosampler vial. For details of the HPLC analyses refer to Fühner (2005).
The Fe concentrations in the ESS and in the extracts of the non-sequential Fe fractionation as well as the concentrations of Na, K, Mg, Ca, Mn, Fe, Al, and Zn in the extracts for the CECeff determination were measured by AAS (Varian SpectrAA 30).
The ferrous iron concentrations in the Ferrozine extracts were measured by photometry at λ = 562 nm based on an extinction coefficient of ɛ = 24.6 mM−1 cm−1.
Following Murphy and Riley (1962), the formation of a molybdate complex was used to determine P by photometry at λ = 712 nm. In preliminary tests the quantity of NaOH or H2SO4 that was necessary to bring the different soil extracts to pH 5.4, the optimal pH for the formation of the blue-coloured complex, was established. Calibrations of the photometric tests were performed separately for each extract type at concentrations between 0 and 20 μM P. The extinction coefficients varied between 15.8 mM−1 cm−1 (resin extractable P) and 17.3 mM−1 cm−1 (HCO3 −-extractable P).
Data analyses
For most investigations, data analyses were performed using the SPSS for Windows 8.0 Statistical Package. Normal distribution and homogeneity of variance were tested using the Shapiro–Wilk test and the Levene test, respectively. If the premises were met for all data sets in an investigation series, mean comparisons of two samples were conducted by t tests, multiple comparisons by one-way ANOVA followed by post hoc Scheffé tests. In all other cases, non-parametric statistics were applied. Two samples were compared here by Mann–Whitney U tests, more than two samples by applying Kruskal–Wallis H tests followed by post hoc pairwise Schaich–Hamerle comparisons performed by a Microsoft EXCEL AddIn programmed in Visual Basic.
SigmaPlot for Windows (version 6.0, Systat Software Inc.) was used for linear and non-linear regression analyses. Data sets were tested here for normality by the Kolmogorov–Smirnov procedure, for constant variance by computing the Spearman rank correlations between the absolute values of the residuals and the observed values of the dependent variables. Durbin–Watson tests were used to check the assumption that the residuals are independent of each other, rejecting the null hypothesis if the Durbin–Watson values deviated from 2 by more than 0.50. In addition to the regression equations, the adjusted coefficients of determination \(r_{{\text{adj}}}^2 \) and the results for one-way ANOVA were recorded.
Results
Plant species composition
C. vulgaris, given the reaction value 1 (strictly calcifuge) by Ellenberg et al. (1992), is present in all CF plots at the main sites and in the CF plots of three of the supplementary sites, covering between <5% and >25% of the individual plots. At site V, it is missing, but according to an information from the nature conservation authority it was recorded there until at least 1993. Therefore, and because of the frequent occurrence of Danthonia decumbens L. (reaction value 3, moderately calcifuge) in the CF plot the site was included into the investigations. D. decumbens also occurs frequently in CF plots at the other sites. The calcifuge moss Pleurozium schreberi (Brid.) Mitt. is present in all CF plots at the main sites but is absent at the supplementary sites. Except for these species, only two other moderately calcifuge species [Luzula campestris (L.) Dc., Polygala vulgaris L.] were found in a few cases (Table 2). On the other hand, calcicole species are numerous at all sites even on the CF plots.
General soil conditions and pH values
CC and CF plots of the main sites differ in several respects (Table 3): CC plots, except for site V, are situated on steeper slopes than CF plots, and the latter tend to have a more northerly orientation. CF soils are deeper (except for site V), and their CaCO3 content is lower. According to the US Soil Taxonomy the soil texture of all plots is silt loam.
Tendentially, the soil pH is lower on the CF plots than at the CC plots, in accordance with the lower CaCO3 contents and lower base saturations. However, the pHH2O was generally high even in CF soils. A pHH2O < 6.0 was found only in one case, and in four of six cases appears a pHH2O > 7.0. The pHKCl varied more strongly. CC soils exhibited values between 7.4 and 6.9. The CF soils fall into two groups: One group with pHKCl values ≤4.8, and another group with pH values ≥6.2.
Although the Co/N ratios were generally very low, they were higher in the CF soils than in the CC soils, except for site V. The concentrations of NO3 − in the ESS of the CC plots always exceeded those of the corresponding CF plots, in median for all study sites 16-fold. Exceptionally high values for the CC plots at site IV and site VI, where soil samples were taken only once, can possibly be attributed to the high temporal variability of the microbial mineralization rate. Nevertheless, the NO3 − concentrations in the plots were quite low, spanning a range of 0.2 to 427 nmol gDW −1 (corresponding to 0.1 to 213.5 μM measured in the ESS).
Several soil parameters were investigated only at the two main sites (Table 3). The CF soils contained less coarse material than the CC soils, and their fine soil density was higher. The volumetric water content Θ, which was measured several times throughout the year, was always lower in the CC soils. The median Θ in the CF soils exceeded that of the CC plots by 71% (site I) and by 23% (site II), respectively. The lowest Θ (<15%) was found at site II after a 3 weeks of drought period in the middle of May (data not shown). However, similar or even lower extreme values are to be expected in the very shallow CC soil at site I.
The DOC in the ESS of the CC plots, which fall below the values of the CF plots, were in accordance with Co and Θ of the fine soil. Lower Co and higher DOC in the CF soils could be due to higher Θ, favouring the microbial degradation of organic material. Based on differences between total and inorganic carbon, no Co could be calculated for the CC plot at site VI. Since the carbonate content of the fine soil at this site is very high, the organic carbon content may be masked by inaccuracy of the inorganic carbon measurement.
The CECeff in the fine soils of the CF plots at site I and site II showed a tendency toward slightly lower values. However, the CECeff in the CF soils of site II was nearly equal to the CECeff of the CC soil at site I (Table 3). The base saturations of the CC plots exceeded only slightly, but significantly, the values of the CF plots.
Fe fractions and soil acidity
No seasonal trend of the pH or of the Fe concentration was observed in the ESS of the main sites in the course of the year (Fig. 1). The CF soil at site I exhibits a much more variable pH than the other soils, in accordance with its much lower CaCO3 content and, consequently, its lower buffering capacity. At the same time, the median Fe concentration of the fine soil in the CF plot at site I exceeds that of the CC soil by about 64%, despite their nearly identical median pH values.
The H2O- and Ferrozine-extractable Fe contents, representing dissolved and easily soluble Fe, generally indicate a higher plant Fe supply at a CF variant compared to the corresponding CC plot. The DTPA, NTA and oxalate extracts show similar patterns, except for two cases (Fig. 2).
Median Fe concentrations in fine soil material \(\left( {\mu {\text{mol}}\;\;{\text{g}}_{{\text{DW}}}^{ - 1} } \right)\) of CC plots (white bars) and CF plots (black bars) of the study sites I–VI extracted with H2O, Ferrozine, DTPA, NTA and sodium oxalate/oxalic acid. Indexes refer to the results of Mann–Whitney U tests for comparisons of CC plots and CF plots at each site (n = 4, n = 8 for H2O and Ferrozine extracts from sites I–II; n.s.P > 0.05, *P ≤ 0.05, ***P < 0.01, P < 0.001)
Differences between the sites are similarly reflected by the various extracts: The CF soils of the sites I, IV and V, characterised by low pHKCl values, generally exhibit relatively high Fe contents, except for the ESS of the CF soil at site I. The Ferrozine-, DTPA- and oxalate-extractable Fe fractions are strongly correlated with the pHKCl (Fig. 3; Table 4). The regressions show that the group of CF soils with pHKCl values <4.8 clearly contrasts with the CC soils and the group of CF soils with higher pHKCl values.
The water soluble Fe fraction deviates from the other fractions as it is more strongly correlated with the pHH2O than with the pHKCl (Fig. 4; Table 4). In accordance with the known dependency of dissolved Fe(III) species from solution pH, all soils in the pHH2O range between 7.0 and 8.0 exhibited very low FeH2O contents, whereas the highest water soluble soil Fe content was found in the CF soil at site IV where the pHH2O was lowest. At the study site I the median pHH2O values of 7.4 (CF) and 8.0 (CC) correspond to an eightfold Fe concentration in the aqueous extracts of the CF soil.
Phosphate fractions
The sequential P extractions for the soils of the study sites I and II do not indicate a higher P availability in the CF plots (Fig. 5). Quite the reverse, the content of the easily accessible Presin significantly exceeds that in the CC soils. The content of NaHCO3-extractable Olsen-P is higher in the CF soil at site I, but lower in the CF soil at site II. At both sites the sum of Presin and PNaHCO3 in the CC soils tends to exceed the content of these fractions in the CF soils. The contents of the less easily accessible NaOH-extractable fraction was higher in the CF soil at site I but not at site II , and the contents of the HCl-soluble fraction was considerably lower at both sites in the CF soils than in the CC soils.
As a result, a comparison of CF soils and CC soils at the supplementary sites was restricted to the P concentrations of the ESS. These concentrations were only significantly higher in CF soil than in CC soil at site IV, whereas at the other sites no significant concentration differences were found (Fig. 6). Again, the P contents of CF soils at some sites were lower than the contents of CC soils at other sites.
P fractions \(\left( {\mu {\text{mol}}\;\;{\text{g}}_{{\text{DW}}}^{ - 1} } \right)\) determined by sequential P analyses of air-dry fine soil from CC plots and CF plots at study sites I and II (means ± SD, n = 16). Indexes refer to the results of Student-t-tests for site-specific CC/CF comparisons (n.s. P > 0.05, **P < 0.01, ***P < 0.01, P < 0.001)
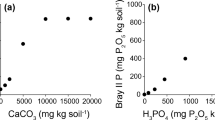
Mean P concentrations \(\left( {\mu {\text{mol}}\;\;{\text{g}}_{{\text{DW}}}^{ - 1} } \right)\) of aqueous extractions of CC soils (white bars) and CF soils (black bars) of study sites I–VI. a Extracts from air-dry ground soil, using H2O and anion exchanger resin (n = 16). b ESS from field-moist sieved soil (n = 4). Indexes refer to the results of Mann–Whitney U tests CC vs. CF (n.s. P > 0.05, *P = 0.050, **P = 0.010, ***P < 0.001)
Discussion
Quantification of plant-available Fe and P in calcareous soils
The Fe pool in the soil includes a multitude of solid and dissolved inorganic and organic fractions of ferric and ferrous iron. Hydrolysis of Fe oxides, Fe hydroxides and Fe oxyhydroxides (denoted here as Fe (oxy/hydr)oxides), chelation of Fe3+, as well as reductive mobilisation and reoxidation of Fe2+ depend on the soil pH. Thus, soil acidity is the key factor determining Fe speciation and, therefore, the Fe solubility in the soil. In principle, Fe solubility is minimal around a pH of 8, i.e. within the buffer range of calcareous soils, and increases with decreasing pH. However, depending on the texture and water content of the soil, the microbial activity and the redox potential, the quality and quantity of inorganic and organic material (i.e. the concentration of Fe complexing compound) can vary, causing different Fe solubilities at a given pH.
The general pH dependency of Fe solubility is reflected by the results of our investigations of the ESS (Fig. 4; Table 4). When the results of all Fe analyses are plotted against the pH (Fig. 7), the course of the regression function is in principal similar to that presented by Lindsay (1979) for inorganic ferric iron. However, since these extracts contain also Fe2+ and organic Fe-complexes, the mean Fe concentrations in the ESS are higher.
Non-linear regression for log c(FeH2O) vs. pHH2O based on all measurements in ESS of CC soils (white symbols) and CF soils (black symbols). Hatching indicates the pH range of minimal solubility of inorganic Fe(III) species according to Lindsay (1979)
In contrast to FeH2O, which depends primarily on the pHH2O, the other Fe fractions show a stronger correlation with pHKCl (Fig. 3; Table 4). This is plausible because they result ultimately from the pH-dependent weathering of Fe minerals, and because the pHKCl reflects the maximal acidity that occurred during the course of weathering in these soils (Ulrich 1986).
Similarly to Fe, the speciation of P in the soil is primarily a function of pH (Lindsay et al. 1989). However, plant P availability is much less correlated with the pH and CaCO3 content of the soil. The precipitation of Ca phosphates and the formation of apatites is promoted by high pH and Ca2+ concentrations, whereas orthophosphate anions can be desorbed from exchange sites of inorganic and organic soil constituents by the high activities of OH− and HCO3 − typical for calcareous soils (Hinsinger 2001). Accordingly, we measured higher mean PH2O contents in CC soils despite higher pH than in CF soils (Fig. 5).
Does Fe and/or P nutrition affect the occurrence of C. vulgaris in semi-arid calcareous grasslands?
At each individual site, the FeH2O content of the CF soil is higher than that of the corresponding CC soil. However, a comparison between the study sites reveals that the upper pH ranges of CF soils and CC soils overlap to a great degree (Table 3), and that the Fe solubility in some CC plots exceeds that in the CF plots at other sites (Fig. 2).
When using Ferrozine as extractant, not only dissolved but also adsorbed and weakly bound Fe2+ ions are determined (Fühner 2005). FeFerro is only loosely correlated with the FeH2O (R 2 = 0.540; Table 4). Nevertheless, it shows a similar distribution pattern: At each site the FeFerro content of the CF plot exceeds that of the corresponding CC plot, but at some sites the CC soils show higher contents of FeFerro than the CF soils at other sites (Fig. 2). The same applies to the fractions of FeDTPA, FeNTA and FeOxal. Thus, the analyses of all Fe fractions in field-moist fine soil do not support the hypothesis that a higher Fe supply enables C. vulgaris and other calcifuge plant species to grow in calcareous grasslands.
No indications of a higher P availability in the CF soils compared to the CC soils were found. On the contrary, the Presin contents, characterising the most easily available fraction, indicate a higher availability in the CC soils of the extensively investigated main sites I and II (Fig. 5). The PNaHCO3 contents and the PNaOH contents exhibit a non-uniform distribution, and the PHCl contents, characterising a fraction which would become available in the long term due to weathering, are much higher in the CC soils. The differences between the P contents in the ESS of the CC soils and CF soils at the supplementary sites III to VI are inconsistent and for the most part insignificant (Fig. 6). They do not provide an argument for a decisive role of P availability in the spatial distribution of C. vulgaris and the moderately calcifuge species at the study sites. Thus, the primary hypothesis that the distribution of C. vulgaris and other calcifuge plant species in calcareous grasslands is influenced by the P supply is not supported by the results.
Secondary effects on plant Fe and P nutrition at the study sites
Even if the contents of the investigated Fe and P fractions do not differ substantially between CC soils and CF soils, interferences with other soil properties could result in different availabilities of these nutrients. Properties which are always higher in CC soils than in CF soils are the CaCO3 and the NO3 − contents, whereas, as measured at the main sites, the volumetric water contents and the soil densities are lower (Table 3).
Higher moisture and higher density reduce soil aeration and can thus increase the reductive formation of dissolved Fe2+. Moreover, higher soil moisture can favour microbial activity and, thus, the dissolution of Fe(III) by organic ligands in CF soils compared to CC soils of similar pH (Fig. 7). Contrary physical conditions in CC soils could reduce the formation of plant available Fe by inhibiting microbial activity, as indicated by the lower DOC and higher Co contents (Table 3). However, in view of the fact that the contents of the various Fe fractions are higher in some CC soils than in some CF soils, this cannot be of general significance for the occurrence of calcifuges at the sites investigated.
In systems saturated with calcite the HCO3 − concentration is positively and the pH negatively correlated with the partial pressure of CO2 (Loeppert 1988). Consequently, plant and microbial respiration can result in a drop of the pH and, in the process, enhanced dissociation of Fe-(oxy/hydr)oxides in the rhizosphere of plants on calcareous soils. Moreover, Fe(III) reduction and formation of organic Fe complexes could be favoured, additionally improving the Fe supply to the plants. However, negative effects could result from the production of HCO3 − in the course of CaCO3 dissolution. HCO3 − can reach high concentrations, especially, if the gas exchange is reduced by higher densities and/or moisture contents of the soil (e.g. Misra and Tyler 1999). Since HCO3 − in combination with its buffering capacity to neutral or slightly alkaline pH interferes with external and internal mechanisms of Fe acquisition in several ways (Doney et al. 1960; Lee and Woolhouse 1969b; Toulon et al. 1992; Mengel 1994, Romera et al. 1997; Römheld 2000), it could, theoretically, result in a reduced Fe supply to plants in CaCO3-rich CC soils compared to CaCO3-poorer CF soils, even at similar soluble Fe contents. Our investigations do not allow a definite decision as to whether such HCO3 − effects play a role in the exclusion of C. vulgaris and the moderately calcifuge species from the CC soils. Lee and Woolhouse (1971) have shown that selected calcifuge species are more susceptible to HCO3 − than the calcicole species selected for comparison. Leake et al. (1990) found that C. vulgaris with Hymenoscyphus ericae as mycorrhizal fungus suffers from decreasing Fe uptake in nutrient solutions at pH 7.0 when the CO3 2− concentrations exceed 2.5 mM. But, special studies into the effects of realistic HCO3 − concentrations on this species have not yet been performed.
The CaCO3 contents of CF soils are still sufficient to keep the pH within the carbonate-buffer range. Concurrently, the higher density as well as the higher mean moisture content could favour the enrichment of HCO3 − in CF soils. As a consequence, it seems unlikely that HCO3 − affects the Fe supply to C. vulgaris in CF soils less than in CC soils. Thus, HCO3 − is probably not decisive for the species’ exclusion from the CC plots.
In addition to HCO3 −, NO3 − is also known to interfere with the Fe utilisation of plants (Mengel 1994). Theoretically, this could be another factor that prevents calcifuge species from growing in CC soils as higher concentrations were always found in those soils than in CF soils. However, apart from the fact that the existence of a higher susceptibility to NO3 − in calcifuge species than in calcicole ones has not yet been established, such an effect is improbable because the absolute NO3 − concentrations in the soil extracts were always in a nanomolar to micromolar range, whereas detrimental effects were established in nutrient solutions with NO3 − concentrations in the millimolar range.
Effects of HCO3 − and NO3 − on the uptake and utilisation of P are not known. Theoretically, anions could improve P availability due to the desorption of phosphates from anion exchangers, and higher soil moisture could improve the diffusive translocation of P to the root surface (Hinsinger 2001). These processes would favour the plant P supply in CF soils. But their practical significance in the case of C. vulgaris is doubtful because this species’ P acquisition is supported by the associated mycorrhiza and the network of mycelial hyphae in the soil that diminishes the relative importance of a diffusive translocation.
Therefore, an exclusion of C. vulgaris from the investigated calcareous soils by Fe or P deficiency appears rather improbable, even if secondary effects are considered.
A scenario of the connections between topography, pedogenesis and the occurrence of C. vulgaris
The CF plots differ from the CC plots by a lower slope and/or a more northerly exposition (Table 3). Consequences of these characteristics, such as lower insolation and probably lower surface run-off and higher soil moisture, have led to a more intense weathering and, thus, to the deeper solum, to the lower contents of CaCO3 and HCl-extractable P, and, at the level of individual sites, to the higher actual and particularly higher potential acidity of the CF soils. A lower evaporative demand and a more continuous soil water availability may have favoured the establishment of C. vulgaris, whereas contrary conditions at CC plots may have prevented moisture-demanding seed germination (Pons 1989) and the establishment of the species’ seedlings, which are very susceptible to drought (Gimingham 1960). Once established, C. vulgaris may have accelerated soil acidification and may have contributed to the phenomenon that unusually low pH values, reflected by potential soil acidities down to pHKCl 4.3 were temporarily reached in these soils (Ulrich 1986) despite the originally high CaCO3 contents. The soil-acidifying effect of C. vulgaris is a well known phenomenon (Grubb et al. 1969; Grubb and Suter 1971).
The current degree of coverage of this species appears insufficient to produce soil acidification down to pH 4.3. However, according to oral information from the local shepherd and the nature conservation representative, the CF plots at sites I and II had a closed and high C. vulgaris cover in former times. In the second half of the last century the cover of C. vulgaris at the study sites declined continuously, coinciding with a decrease in grazing intensity and a simultaneous increase of aerial N input as well as increasing competition from upcoming bushes, which are now removed periodically by public conservation actions. Competition from grasses and bushes, promoted by atmospheric N deposition, is probably the main cause of the still ongoing decline of C. vulgaris. Increased drought sensitivity as a consequence of high N supply (Gordon et al. 1999) could be additionally involved. Controlled burning as used in heathland management on acid soils and moorland under more oceanic climates in order to reduce ecosystem N pool and N turnover (e.g. Nilsen et al. 2005; Pilkington et al. 2007), was not applied at our study sites. Damages to C. vulgaris through detrimental effects of the increased N supply on its ericoid mycorrhiza is unlikely because Johansson (2000) found no effects of fertilizations as high as 70 kg N ha−1 year−1 in the form of ammonium nitrate. Inhibition of ericoid mycorrhizal infection of C. vulgaris roots in moorland peat by ammonium sulphate and nitric acid fertilization, observed by Yesmin et al. (1996), were due to simultaneous soil acidification and are not relevant at the sites investigated here.
Under the current conditions, buffering processes obviously exceed acidifying processes in the CF plots, resulting in a pHH2O well above the pHKCl. But the latter provides evidence of much stronger acidification of the CF soils in the past, exceeding the buffering rate for a long time (Ulrich 1986). Since this is most probably an effect of C. vulgaris, the causal chain of our initial hypothesis becomes reversed: Higher soil acidity and higher Fe availability are consequences of and not preconditions for the establishment of C. vulgaris, at least in the semi-arid calcareous grasslands of the main sites. But, a similar development can be assumed at the other CF plots.
We assume, therefore, that local moisture conditions were decisive for the initial establishment of C. vulgaris and that weathering remained much weaker at the CC plots because the establishment of C. vulgaris was prevented there by drought. The establishment of other moderately calcifuge species in the CF plots could then be favoured by the soil acidifying effect of C. vulgaris.
In the present state, C. vulgaris occurs at our study sites on soils with pHH2O values up to 7.9 and with pHKCl values up to 6.6. Correspondingly, the base saturation of CF soils amounts to 99.5%. Its growth under these conditions emphasises that the calcifuge distribution of this species is not caused by a physiological inability to cope with high pH and high Ca contents of the substrate. Apparently, C. vulgaris, regarded as a strictly calcifuge species and given the reaction value 1 by Ellenberg et al. (1992), is able to grow on calcareous soils with high pH values, even if the species has its main occurrence on Ca-poor and very acidic soils. Therefore, the reaction value 1 (strong acidity indicator, never occurring on slightly acid to alkaline soils) does not appear to be appropriate.
References
Borggaard OK (1988) Phase identification by selective dissolution techniques. In: Stucki JW, Goodman BA, Schwertmann U (eds) Iron in soils and clay minerals. Reidel, Dordrecht, pp 83–98
Campbell AS, Schwertmann U (1984) Iron oxide mineralogy of placic horizons. J Soil Sci 35:569–582 doi:10.1111/j.1365-2389.1984.tb00614.x
Doney RC, Smith RL, Wiebe HH (1960) Effects of various levels of bicarbonate, phosphorus and pH on the translocation of foliar-applied iron in plants. Soil Sci 89:269–275 doi:10.1097/00010694-196005000-00007
Dušek L (1995) The effect of cadmium on the activity of nitrifying populations in two different grassland soils. Plant Soil 177:43–53 doi:10.1007/BF00010336
Ellenberg H, Weber HE, Düll R, Wirth V, Werner W, Paulissen D (1992) Zeigerwerte von Pflanzen in Mitteleuropa, 2nd edn. Goltze, Göttingen
Falkengren-Grerup U, Quist ME, Tyler G (1995) Relative importance of exchangeable and soil solution cation concentrations to the distribution of vascular plants. Environ Exp Bot 35:9–15 doi:10.1016/0098-8472(94)00039-8
FAO (2006) World Reference Base for soil resources (WRB), Resources Reports 103. FAO, Rome
Fühner C (2005) Das Auftreten acidophiler/calcifuger Pflanzenarten in Kalk-Halbtrockenrasen. PhD thesis, University of Göttingen, Dep. Ecology and Ecosystems Research
Gimingham CH (1960) Biological flora of the British isles: Calluna vulgaris (L.) HULL. J Ecol 48:455–483 doi:10.2307/2257528
Gordon C, Woodin SJ, Alexander IJ, Mullins CE (1999) Effects of increased temperature, drought and nitrogen supply on two upland perennials of contrasting functional type: Calluna vulgaris and Pteridium aquilinum. New Phytol 142:243–258 doi:10.1046/j.1469-8137.1999.00399.x
Gries D, Runge M (1995) Responses of calcicole and calcifuge Poaceae species to iron limiting conditions. Bot Acta 108:482–489
Grime JP (1963) Factors determining the occurrence of calcifuge species on shallow soils over calcareous substrata. J Ecol 51:375–390 doi:10.2307/2257691
Grime JP, Hodgson JG (1969) An investigation of the ecological significance of lime-chlorosis by means of large-scale comparative experiments. In: Rorison RH (ed) Ecological aspects of the mineral nutrition of plants. Blackwell, Oxford, pp 67–99
Grubb PJ, Suter MB (1971) The mechanism of soil acidification by Calluna and Ulex and the significance for conservation. In: Duffey E, Watt AS (eds) The scientific management of plant and animal communities for conservation. Blackwell, Oxford, pp 115–133
Grubb PJ, Green HE, Merrifield RCJ (1969) The ecology of chalk heath: its relevance to the calcicole-calcifuge and soil acidification problems. J Ecol 57:175–212 doi:10.2307/2258215
Hinsinger P (2001) Bioavailability of soil inorganic P in the rhizosphere as affected by root-induced chemical changes: a review. Plant Soil 237:173–195 doi:10.1023/A:1013351617532
Johansson M (2000) The influence of ammonium nitrate on the root growth and ericoid mycorrhizal colonization of Calluna vulgaris (L.) Hull from a Danish heathland. Oecologia 123:418–424 doi:10.1007/s004420051029
Koperski M, Sauer M, Braun W, Gradstein SR (2000) Referenzliste der Moose Deutschlands. Landwirtschaftsverlag, Münster
Kosegarten HU, Hoffmann B, Mengel K (1999) Apoplastic pH and Fe3+ reduction in intact sunflower leaves. Plant Physiol 121:1069–1079 doi:10.1104/pp.121.4.1069
Leake JR, Shaw G, Read DJ (1990) The biology of mycorrhiza in the Ericaceae—XVI. Mycorrhiza and iron uptake in Calluna vulgaris (L.) Hull in the presence of two calcium salts. New Phytol 114:651–657 doi:10.1111/j.1469-8137.1990.tb00436.x
Lee JA, Woolhouse HW (1969a) A comparative study of bicarbonate inhibition of root growth in calcicole and calcifuge grasses. New Phytol 68:1–11 doi:10.1111/j.1469-8137.1969.tb06413.x
Lee JA, Woolhouse HW (1969b) Root growth and dark fixation of carbon dioxide in calcicoles and calcifuges. New Phytol 68:247–255 doi:10.1111/j.1469-8137.1969.tb06437.x
Lee JA, Woolhouse HW (1971) The relationship of compartmentation of organic acid metabolism to bicarbonate ion sensitivity of root growth in calcicoles and calcifuges. New Phytol 70:103–111 doi:10.1111/j.1469-8137.1971.tb02515.x
Lindsay WL (1979) Chemical equilibria in soils. Wiley, New York
Lindsay WL, Norvell WA (1978) Development of a DTPA soil test for zinc, iron, manganese, and copper. Soil Sci Soc Am J 42:421–428
Lindsay WL, Vlek PLG, Chien SH (1989) Phosphate minerals. In: Dixon JB, Weed SB (eds) Minerals in soil environment. Soil Science Society of America, Madison, pp 1089–1130
Loeppert RH (1988) Chemistry of iron in calcareous systems. In: Stucki JW, Goodman BA, Schwertmann U (eds) Iron in soils and clay minerals. Reidel, Dordrecht, pp 689–713
Loeppert RH, Suarez DL (1996) Carbonate and gypsum. In: Sparks DL, Page AL, Helmke PA, Loeppert RH, Soltanpour PN, Tabatabai MA, Johnson CT, Sumner ME (eds) Methods of soil analysis. Part 3: Chemical methods. SSSA Special Publication No. 5, Soil Science Society of America, Madison, pp 437–475
Ludwig B, Meiwes KJ, Khanna P, Gehlen R, Fortmann H, Hildebrand EE (1999) Comparison of different laboratory methods with lysimetry for soil solution composition—experimental and model results. J Plant Nutr Soil Sci 162:343–351 doi:10.1002/(SICI)1522-2624(199906)162:3<343::AID-JPLN343>3.0.CO;2-E
Mengel K (1994) Iron availability in plant tissues—iron chlorosis on calcareous soils. Plant Soil 165:275–283 doi:10.1007/BF00008070
Misra A, Tyler G (1999) Influence of soil moisture on soil solution chemistry and concentrations of minerals in the calcicoles Phleum phleoides and Veronica spicata grown on a limestone soil. Ann Bot (Lond) 84:401–410 doi:10.1006/anbo.1999.0941
Murphy J, Riley JP (1962) A modified single solution method for the determination of phosphate in natural waters. Anal Chim Acta 27:31–36 doi:10.1016/S0003-2670(00)88444-5
Niklaus PA (1998) Soil microbial effects of elevated CO2 in calcareous grassland. Glob Chang Biol 4:451–458 doi:10.1046/j.1365-2486.1998.00166.x
Niklaus PA, Falloon P (2006) Estimating soil carbon sequestration under elevated CO2 by combining carbon isotope labelling with soil carbon cycle modelling. Glob Chang Biol 12:1909–1921 doi:10.1111/j.1365-2486.2006.01215.x
Nilsen LS, Johansen L, Velle LG (2005) Early stages of Calluna vulgaris regeneration after burning of coastal heath in central Norway. Appl Veg Sci 8:57–64 doi:10.1658/1402-2001(2005)008[0057:ESOCVR]2.0.CO;2
Pilkington MG, Caporn SJM, Carroll JA, Cresswell N, Phoenix GK, Lee JA et al (2007) Impacts of burning and increased nitrogen deposition on nitrogen pools and leaching in an upland moor. J Ecol 95:1195–1207 doi:10.1111/j.1365-2745.2007.01292.x
Pons TL (1989) Dormancy, germination and mortality of seeds in heathland and inland sand dunes. Acta Bot Neerl 38:327–336
Romera FJ, Alcantara E, de la Guardia MD (1997) Influence of bicarbonate and metal ions on the development of root Fe(III) reducing capacity by Fe-deficient cucumber (Cucumis sativus) plants. Physiol Plant 101:143–148 doi:10.1111/j.1399-3054.1997.tb01830.x
Römheld V (2000) The chlorosis paradox: Fe inactivation as a secondary event in chlorotic leaves of grapevine. J Plant Nutr 23:1629–1643
Snaydon RW, Bradshaw AD (1962) Differences between natural populations of Trifolium repens L. in response to mineral nutrients—I. Phosphate. J Exp Bot 13:422–434 doi:10.1093/jxb/13.3.422
Ström L (1997) Root exudation of organic acids: importance to nutrient availability and the calcifuge and calcicole behaviour of plants. Oikos 80:459–466 doi:10.2307/3546618
Ström L, Olsson T, Tyler G (1994) Differences between calcifuge and acidifuge plants in root exudation of low-molecular organic acids. Plant Soil 167:239–245 doi:10.1007/BF00007950
Susin S, Abadia A, Gonzales-Reyes JA, Lucena JJ, Abadia J (1996) The pH requirement for in vivo activity of the iron deficiency-induced “turbo” ferric chelate reductase. Plant Physiol 110:111–123
Tiessen H, Moir JO (1993) Characterization of available P by sequential extraction. In: Carter MR (ed) Soil sampling and methods of analysis. Lewis Publishers, Boca Raton, pp 75–86
Toulon V, Sentenac H, Thibaud J-B, Davidian JC, Moulineau C, Grignon C (1992) Role of apoplast acidification by the H+ pump. Planta 186:212–218 doi:10.1007/BF00196250
Tyler G (1992) Inability to solubilize phosphate in limestone soils—key factor controlling calcifuge habit of plants. Plant Soil 145:65–70 doi:10.1007/BF00009542
Tyler G (1994) A new approach to understanding the calcifuge habit of plants. Ann Bot (Lond) 73:327–330 doi:10.1006/anbo.1994.1038
Tyler G, Ström L (1995) Differing organic acid exudation pattern explains calcifuge and acidifuge behaviour of plants. Ann Bot (Lond) 75:75–78 doi:10.1016/S0305-7364(05)80011-3
Ulrich B (1986) Natural and anthropogenic components of soil acidification. Z Pflanzenernahr Bodenkd 149:702–717 doi:10.1002/jpln.19861490607
Wisskirchen R, Häupler H (1998) Standardliste der Farn-und Blütenpflanzen Deutschlands. Ulmer, Stuttgart
Yesmin L, Gammack SM, Cresser MS (1996) Effects of atmospheric nitrogen deposition on ericoid mycorrhizal infection of Calluna vulgaris growing in peat. Appl Soil Ecol 4:49–60 doi:10.1016/0929-1393(96)00099-6
Yuan G, Lavkulich LM, Wang C (1993) A method for estimating organic bound iron and aluminum contents in soils. Commun Soil Sci Plant Anal 24:1333–1343
Zohlen A, Tyler G (2000) Immobilization of tissue iron on calcareous soil: differences between calcicole and calcifuge plants. Oikos 89:95–106 doi:10.1034/j.1600-0706.2000.890110.x
Zohlen A, Tyler G (2004) Soluble inorganic tissue phosphorus and calcicole-calcifuge behaviour of plants. Ann Bot (Lond) 94:427–432 doi:10.1093/aob/mch162
Acknowledgments
We would like to thank Magdolna Weller and Simone Klatt for excellent technical assistance. Thanks are also due to Dirk Gries and two anonymous reviewers for helpful comments on the manuscript. This work was supported by a grant from the Deutsche Forschungsgemeinschaft (Ru 136/10-1, 10-2).
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: Philippe Hinsinger.
Rights and permissions
Open Access This is an open access article distributed under the terms of the Creative Commons Attribution Noncommercial License (https://creativecommons.org/licenses/by-nc/2.0), which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
About this article
Cite this article
Fühner, C., Runge, M. Are Fe and P availabilities involved in determining the occurrence and distribution of Calluna vulgaris (L.) Hull in semi-arid grasslands on calcareous soils?. Plant Soil 316, 161–176 (2009). https://doi.org/10.1007/s11104-008-9768-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11104-008-9768-0