Abstract
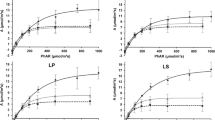
Populus spp. are among the fast-growing tree species most prone to water deficit. The use of drought-resistant clones allows mitigating the negative effects of water deficit on the establishment and the productivity of unstable irrigated plantations in dryland areas. We evaluated the responses of four Populus × canadensis (‘Conti 12’, ‘Guardi’, ‘I-214’ and ‘I45/51’) and four Populus deltoides (‘Stoneville 67’, ‘Catfish 2’, ‘Dvina’ and ‘Australiano 129/60’) clones to a short-term water stress. Three water treatments were imposed on 6-month-old plants: well-watered (WW), 5 days of irrigation withdrawal followed by rewatering (−5 + RW), and 9 days of irrigation withdrawal followed by rewatering (−9 + RW). Populus clones presented different strategies to face water deficit, resulting in variable effects on plant growth. The small-leaved clone ‘Australiano 129/60’ showed strong stomatal control and maintained a high increase in leaf area during the water-shortage period. These strategies were associated with high relative growth under both water-shortage treatments. ‘Australiano 129/60’ showed to be a drought-resistant clone with the highest growth under both water-shortage treatments, appearing to be a promising clone for production in environments prone to short-term water stress.







Similar content being viewed by others
Data availability
The datasets generated during and/or analyzed during the current study are available in the OSF Repository.
Code availability
References
Arneth A, Denton F, Agus F (2019) Framing and context. Climate change and land: an IPCC special report on climate change, desertification, land degradation, sustainable land management, food security, and greenhouse gas fluxes in terrestrial ecosystems. Intergovernmental Panel on Climate Change (IPCC), Geneva, pp 1–98
Attia Z, Domec JC, Oren R, Way DA, Moshelion M (2015) Growth and physiological responses of isohydric and anisohydric poplars to drought. J Exp Bot 66:4373–4381. https://doi.org/10.1093/jxb/erv195
Blum A (2005) Drought resistance, water-use efficiency, and yield potential—are they compatible, dissonant, or mutually exclusive? Aust J Agric Res 56(11):1159–1168
Bogeat-Triboulot MB, Brosché M, Renaut J, Jouve L, Le Thiec D, Fayyaz P, Vinocur B, Witters E, Laukens K, Teichmann T, Altman A, Hausman JF, Polle A, Kangasjärvi J, Dreyer E (2007) Gradual soil water depletion results in reversible changes of gene expression, protein profiles, ecophysiology, and growth performance in Populus euphratica, a poplar growing in arid regions. Plant Physiol 143:876–892. https://doi.org/10.1104/pp.106.088708
Braatne JH, Hinckley TM, Stettler RF (1992) Influence of soil water on the physiological and morphological components of plant water balance in Populus trichocarpa, Populus deltoides and their F1 hybrids. Tree Physiol 11:325–339. https://doi.org/10.1093/treephys/11.4.325
Calderón AD (2010) Forestación con álamos para la obtención de madera de calidad. Cátedra de Dasonomía—Facultad de Ciencias Agrarias, UNCuyo, Mendoza.
Chen S, Wang S, Altman A, Hüttermann A (1997) Genotypic variation in drought tolerance of poplar in relation to abscisic acid. Tree Physiol 17:797–803. https://doi.org/10.1093/treephys/17.12.797
Di Rienzo JA, Guzmán AW, Casanoves F (2002) A multiple-comparisons method based on the distribution of the root node distance of a binary tree. J Agric Biol Environ Stat 7:129–142. https://doi.org/10.1198/10857110260141193
Di Rienzo JA, Casanoves F, Balzarini MG, Gonzalez L, Tablada M, Robledo CW (2018) Infostat versión 2018. Centro de Transferencia InfoSat, FCA, Universidad Nacional de Córdoba, Córdoba
Dunlap JM, Stettler RF, Heilman PE (1995) Genetic variation and productivity of Populus trichocarpa and its hybrids. VIII. Leaf and crown morphology of native P. trichocarpa clones from four river valleys in Washington. Can J for Res 25:1710–1724. https://doi.org/10.1139/x95-185
Ewers BE, Oren R (2000) Analyses of assumptions and errors in the calculation of stomatal conductance from sap flux measurements. Tree Physiol 20:579–589. https://doi.org/10.1093/treephys/20.9.579
Fichot R, Barigah TS, Chamaillard S, Le Thiec D, Laurans F, Cochard H, Brignoolas F (2010) Common trade-offs between xylem resistance to cavitation and other physiological traits do not hold among unrelated Populus deltoides × Populus nigra hybrids. Plant Cell Environ 33:1553–1568. https://doi.org/10.1111/j.1365-3040.2010.02164.x
Fichot R, Brignolas F, Cochard H, Ceulemans R (2015) Vulnerability to drought-induced cavitation in poplars: synthesis and future opportunities. Plant Cell Environ 38:1233–1251. https://doi.org/10.1111/pce.12491
Franks PJ, Drake PL, Froend RH (2007) Anisohydric but isohydrodynamic: seasonally constant plant water potential gradient explained by a stomatal control mechanism incorporating variable plant hydraulic conductance. Plant Cell Environ 30(1):19–30
Giovannelli A, Deslauriers A, Fragnelli G, Scaletti L, Castro G, Rossi S, Crivellaro A (2007) Evaluation of drought response of two poplar clones (Populus x canadensis Mönch “I-214” and P. deltoides Marsh. ’Dvina’) through high resolution analysis of stem growth. J Exp Bot 58:2673–2683. https://doi.org/10.1093/jxb/erm117
Guarnaschelli B, Garau M, Cortizo S, Alvares J, Lemcoff JH (2011) Respuestas diferenciales a la sequía en clones de Populus deltoides cultivados en el Delta del Paraná. In: Terc Congr Int Salicaceas en Argentina. p 9
Guo XY, Zhang XS, Huang ZY (2010) Drought tolerance in three hybrid poplar clones submitted to different watering regimes. J Plant Ecol 3:79–87. https://doi.org/10.1093/jpe/rtq007
Levitt J (1980) Responses of plants to environmental stresses. Vol. II. Water, radiation, salt, other stresses, 2nd edn. Academic Press Inc., New York, p 607
Marlats RM, Senisterra GE, Marquina JL, Ciocchini GR (2009) Populus spp.: Supervivencia y crecimiento en clones implantados en Buenos Aires, Argentina. Revista de La Facultad de Ciencias Agrarias 41(1):77–84
Marron N, Delay D, Petit JM, Dreyer W, Kahlem G, Delmotte FM, Brignolas F (2002) Physiological traits of two Populus x euramericana clones, Luisa Avanzo and Dorskamp, during a water stress and re-watering cycle. Tree Physiol 22:849–858. https://doi.org/10.1093/treephys/22.12.849
Marron N, Dreyer E, Boudouresque E, Delay D, Petit JM, Delmotte FM, Brignolas F (2003) Impact of successive drought and re-watering cycles on growth and specific leaf area of two Populus x canadensis (Moench) clones, “Dorskamp” and “Luisa_Avanzo.” Tree Physiol 23:1225–1235. https://doi.org/10.1093/treephys/23.18.1225
Marron N, Villar M, Dreyer E, Delay D, Boudouresque E, Petit JM, Delmotte FM, Guehl JM, Brignolas F (2005) Diversity of leaf traits related to productivity in 31 Populus deltoides x Populus nigra clones. Tree Physiol 25:425–435. https://doi.org/10.1093/treephys/25.4.425
McDowell NG (2011) Mechanisms linking drought, hydraulics, carbon metabolism, and vegetation mortality. Plant Physiol 155(3):1051–1059
McDowell NG, Pockman WT, Allen CD, Breshears DD, Cobb N, Kolb T, Plaut J, Sperry J, West A, Williams DG et al (2008) Mechanisms of plant survival and mortality during drought: why do some plants survive while others succumb to drought? New Phytol 178:719–739
McKown AD, Cochard H, Sack L (2010) Decoding leaf hydraulics with a spatially explicit model: principles of venation architecture and implications for its evolution. Am Nat 175:447–460. https://doi.org/10.1086/650721
Monclus R, Dreyer E, Delmotte FM, Villar M, Delay D, Boudouresque R, Petit JM, Marron N, Bréchet C, Brignolas F (2005) Productivity, leaf traits and carbon isotope discrimination in 29 Populus deltoides x P. nigra clones. New Phytol 167:53–62. https://doi.org/10.1111/j.1469-8137.2005.01407.x
Monclus R, Dreyer E, Villar M, Delmotte FM, Delay D, Petit JM, Barbaroux C, Le Thiec D, Bréchet C, Brignolas F (2006) Impact of drought on productivity and water use efficiency in 29 genotypes of Populus deltoides x Populus nigra. New Phytol 169(4):765–777. https://doi.org/10.1111/j.1469-8137.2005.01630.x
Pallardy SG (2008) Physiology of woody plants. Elsevier, Amsterdam
Pearce DW, Millard S, Bray DF, Rood SB (2005) Stomatal characteristics of riparian poplar species in a semi-arid environment. Tree Physiol 26:211–218. https://doi.org/10.1093/treephys/26.2.211
Raven JA (2002) Selection pressures on stomatal evolution. New Phytol 153:371
Roden J, Van Volkenburgh E, Hinckley TM (1990) Cellular basis for limitation of poplar leaf growth by water deficit. Tree Physiol 6:211–219. https://doi.org/10.1093/treephys/6.2.211
Rood SB, Patiño S, Coombs K, Tyree MT (2000) Branch sacrifice: cavitation-associated drought adaptation of riparian cottonwoods. Trees 14:248–257
Scholander PF, Hammel HT, Bradstreet ED, Hemmingsen EA (1965) Sap pressure in vascular plants. Science 148:339–346. https://doi.org/10.1126/science.148.3668.339
Schreiber SG, Hacke UG, Chamberland S, Lowe CW, Kamelchuk D, Bräutigam K, Campbell MM, ThomasBR, (2016) Leaf size serves as a proxy for xylem vulnerability to cavitation in plantation trees. Plant Cell Environ 39:272–281. https://doi.org/10.1111/pce.12611
Scoffoni C, Rawls M, Mckown A, Cochard H, Sack L (2011) Decline of leaf hydraulic conductance with dehydration: relationship to leaf size and venation architecture. Plant Physiol 156:832–843. https://doi.org/10.1104/pp.111.173856
Silim S, Nash R, Reynard D, White B, Schroeder W (2009) Leaf gas exchange and water potential responses to drought in nine poplar (Populus spp.) clones with contrasting drought tolerance. Trees 23:959–969. https://doi.org/10.1007/s00468-009-0338-8
Sparks JP, Black RA (1999) Regulation of water loss in populations of Populus trichocarpa: the role of stomatal control in preventing xylem cavitation. Tree Physiol 19(7):453–459
Tschaplinski TJ, Tuskan GA, Gunderson CA (1994) Water-stress of black and eastern cottonwood clones and four hybrid progeny. I. Growth, water relations, and gas exchange. Can J for Res 24:364–371. https://doi.org/10.1139/x94-049
Viger M, Smith HK, Cohen D, Dewoody J, Trewin H, Steenackers M, Bastien C, Taylor G (2016) Adaptive mechanisms and genomic plasticity for drought tolerance identifed in European black poplar (Populus nigra L.). Tree Physiol. https://doi.org/10.1093/treephys/tpw017
Acknowledgements
The authors are very grateful to Hugo DeBandi, Gualberto Zalazar, Nicolás Giuffre and Gabriel Zalazar for their invaluable help in maintaining the assay and carrying out the measurements.
Funding
This research received funding from Ministerio de Agricultura, Ganadería y Pesca of Argentina under Grant Agreement No PIA 14004 to I.A.M.
Author information
Authors and Affiliations
Contributions
Conceptualization: CVG, CVG and IAM; Methodology: EAR, AG and LNB; Formal analysis and investigation: EAR and AG; Writing—original draft preparation: EAR and CVG; Writing—review and editing: IAM, CVG, AG and CVG; Funding acquisition: CVG, CVG and IAM; Resources: IAM, CVG; Supervision: CVG, CVG and IAM.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflict of interest to declare that are relevant to the content of this article.
Ethical approval
'Not applicable'.
Consent to participate
'Not applicable'.
Consent for publication
All authors give their informed consent to this publication and its content.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Rovida Kojima, E.A., Gonzalez, C.V., Mundo, I.A. et al. Differential responses of Populus deltoides and Populus × canadensis clones to short-term water deficit. New Forests 54, 421–437 (2023). https://doi.org/10.1007/s11056-022-09929-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11056-022-09929-7