Mulberi Leaves
Morus alba L.
Moraceae
DEFINITION
Mulberi leaves consist of the powder of dried leaves of Morus alba L. (Moraceae).
SYNONYM
Morus alba f. alba., Morus alba f. skeletoniana (C.K.Schneid.) Rehder., Morus alba var. atropurpurea (Roxb.) Bureau., Morus alba var. bungeana Bureau., Morus alba var. emarginata Y.B.Wu., Morus alba var. latifolia (Poir.) Bureau., Morus alba var. multicaulis (Perr.) Loudon., Morus alba var. skeletoniana C.K.Schneid., Morus alba var. tatarica (L.) Ser., Morus atropurpurea Roxb., Morus chinensis Lodd., Morus constantinopolitana hort.ex Poir., Morus cucullata Bonaf., Morus dulcis Royle., Morus guzziola hort.ex Steud., Morus heterophylla Loudon., Morus intermedia Perr., Morus kaki hort. ex Lavallée., Morus levasseurei hort.exLavallée., Morus lhou Koidz., Morus lucida hort. ex Loudon., Morus mariettii hort.ex Steud., Morus multicaulis Perr., Morus nana Audib.ex Loisel., Morus nigriformis Koidz., Morus patavia Audib.ex Dippel., Morus patavina hort.ex Spach., Morus pumila Balb., Morus romana Lodd.ex Spach., Morus tokwa hort.ex K.Koch., Morus venosa Delileex Spach [1].
VERNACULAR NAMES
Mulberry, white mulberry, white mulberry tree (English); mulberi, tut, bebesaram, besaram, kayu besar (Malay); Sang Shèn Ye (Chinese); Kambli chedi (Tamil) [2, 3, 4, 5].
CHARACTER
| Colour | Green |
| Odour | Odourless |
| Taste | Slightly bitter and astringent [6, 7] |
IDENTIFICATION
Plant Morphology
M. alba is a small to medium-sized tree, 3 to 15 m tall, bole up to 70 cm in diameter. Bark surface dark greyish brown, with horizontal lenticels. Branches finely hairy. Stipules lanceolate, 2-3.5 cm, densely covered with short pubescence. Leaves ovate to broadly ovate, 5-16 cm in length, 4-12 cm in width, rounded to shallowly cordate at base, acute to acuminate at apex, pubescent on the main veins, with a slender, 1-5.5 cm long petiole. Abaxially sparsely pubescent along midvein or in tufts in axil of midvein and primary lateral veins, adaxially bright green and glabrous. Male catkins pendulous, 2-3.5 cm, densely white hairy. Female catkins 1-2 cm, pubescent; peduncle 5-10 mm, pubescent. Male flowers: calyx lobes pale green, broadly elliptic; filaments inflexed in bud; anthers 2-loculed, globose to reniform. Female flowers sessile; calyx lobes ovoid, with marginal hairs; ovary sessile, ovoid; style absent; stigmas with mastoid-like protuberance, branches divergent, papillose. Fruit blackish purple when mature, 1.5-2.5 cm long, ovoid to ellipsoid [8, 9].
Microscopy
Powdered dried leaves consist of adaxial epidermis cells with straight anticlinal wall and abaxial epidermis cells with sinuous anticlinal wall, anomocytic and paracytic stomata cells; abundant of calcium oxalate crystals (solitary and druse forms). The presence of cystolith calcium carbonate and oil globules are also observed. Other features observed include simple, multicellular trichome, cone-shaped trichome; fiber; parenchyma cells; scalariform, annular, pitted, and spiral vessels.
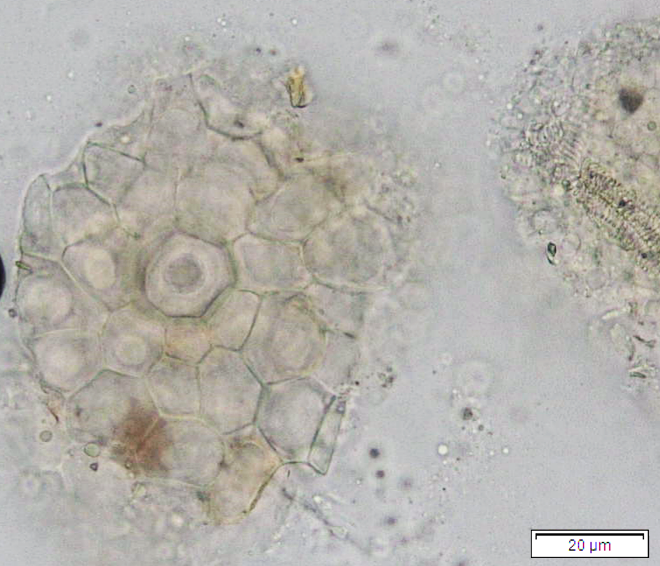
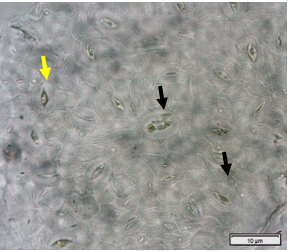
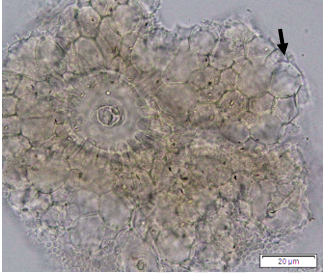
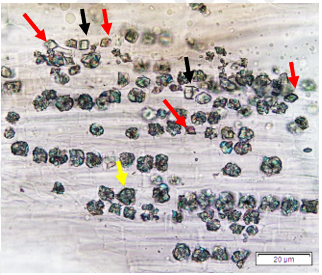
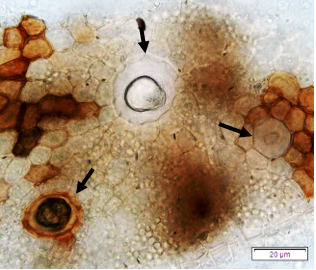


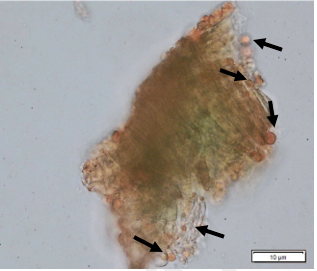

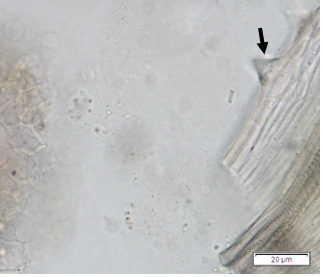
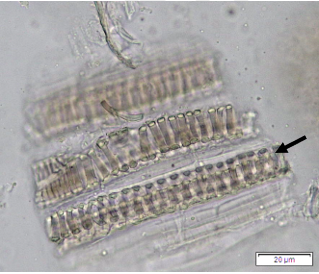


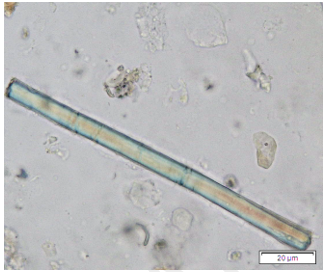
Figure 2 : Microscopic characters of Morus alba dried leaf powder of 0.355 mm size. (a) Adaxial epidermal cells (upper surface) with straight anticlinal wall (magnification 20×); (b) abaxial epidermal cells (lower surface) with curved to wavy anticlinal wall and heterostomatic (anomocytic stomata (black arrow), paracytic stomata (yellow arrow)) (magnification 40×); (c) parenchyma cells (magnification 20×); (d) calcium oxalate crystals; druses (yellow arrow) and solitary rhombus (red arrow), and rectangle (black arrow) (magnification 20×); (e) cystolith structures on epidermis cells (upper view) (black arrow) (magnification 20×); (f) cystolith structure on palisade mesophyll fragment (in front view) (black arrow) (magnification 20×); (g) oil globules (black arrow) (magnification 20×); (h) oil globules in Sudan red (III) (black arrow) (magnification 20×); (i) simple, multicellular trichome (magnification 20×); (j) simple, cone-shaped trichome (black arrow) (magnification 20×); (k) spiral vessel (black arrow) (magnification 20×); (l) scalariform (yellow arrow) and annular (red arrow) vessels (magnification 20×); (m) pitted vessel (magnification 20×); (n) fibre (magnification 20×). [Scale bars: a, c, d, e, f, g, i, j, k, l, m, n = 20 μm; b, h= 10 μm]
Colour Test
Observation of solution after treatment with reagent:
| Test for the presence of phenolics | Bluish green |
High Performance Thin Layer Chromatography (TLC)
| Test Solution | Weigh about 1.0 g of M. alba dried leaf powder of 0.355 mm size in 100 mL conical flask. Add 10 mL of water: ethanol (1:4) and sonicate the mixture for 15 minutes. Centrifuge the mixture and collect the supernatant. |
| Standard solution |
Dissolve rutin standard [CAS no.: 153-18-4] in methanol to produce a standard concentration of 0.2 mg/mL. Dissolve chlorogenic acid standard [CAS no.: 327-97-9] in water to produce a standard concentration of 0.2 mg/mL. [Vortex standard for 10 sec and sonicate for 15 min at room temperature before use.] |
| Stationary Phase | HPTLC Silica gel 60 F254, 10 x 10 cm |
| Mobile phase |
Ethyl acetate : water : formic acid : acetic acid (35: 8 : 3 : 3) (v/v/v/v) |
| Application |
|
| Development distance | 8 cm |
| Drying | Air dry |
| Detection |
|


Figure 3 : HPTLC chromatogram of rutin standard (S1), chlorogenic standard (S2), ethanol (80%) extract of M. alba dried leaf powder (L) observed under (a) UV at 254 nm before derivatization of NP-PEG, and (b) UV at 366 nm after derivatization of NP-PEG.
High Performance Liquid Chromatography (HPLC)
| Test solution | Weigh about 1.0 g of Morus alba dried leaf powder of 0.355 mm size in 100 mL conical flask. Add 10 mL of water: ethanol (1:4) and sonicate the mixture for 15 minutes. Centrifuge the mixture and collect the supernatant. Take 100 µL supernatant, then dilute with 100 µL water. | |||||||||||||||||||||||||||||||||
| Standard solution |
Dissolve rutin standard [CAS no.: 153-18-4] in methanol to give a standard concentration of 0.2 mg/mL. Dissolve chlorogenic acid standard [CAS no.: 327-97-9] in water to give a standard concentration of 0.2 mg/mL. [Vortex standard for 10 sec and sonicate for 15 min at room temperature before use.] |
|||||||||||||||||||||||||||||||||
| Chromatographic system |
Detector: 330 nm Column: C18 (150 x 4.6 mm) (Zorbax SB-C18 150 X 4.6 mm, 3.5 µm) Column oven temperature: 25˚C Flow rate: 1 mL/min Injection volume: 20 µL |
|||||||||||||||||||||||||||||||||
| Mobile phase (gradient mode) |
|
|||||||||||||||||||||||||||||||||
| System suitability requirements |
Perform at least five replicate injections of the standard solutions (0.2 mg/mL). The requirements of the system suitability parameters are as follow:
|
|||||||||||||||||||||||||||||||||
| Acceptance criteria |
|

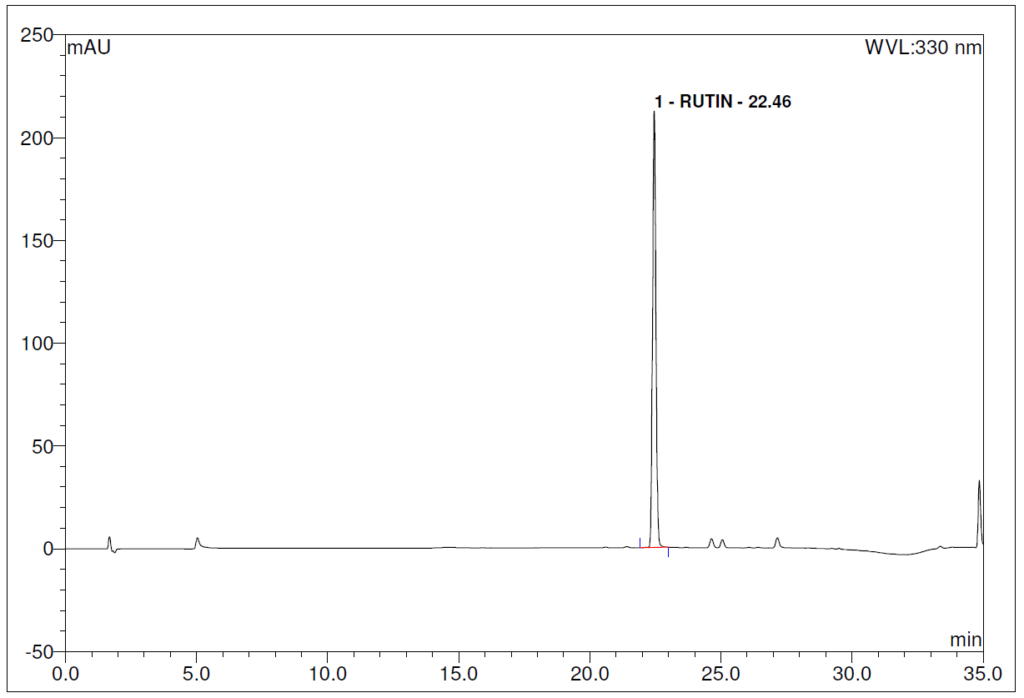
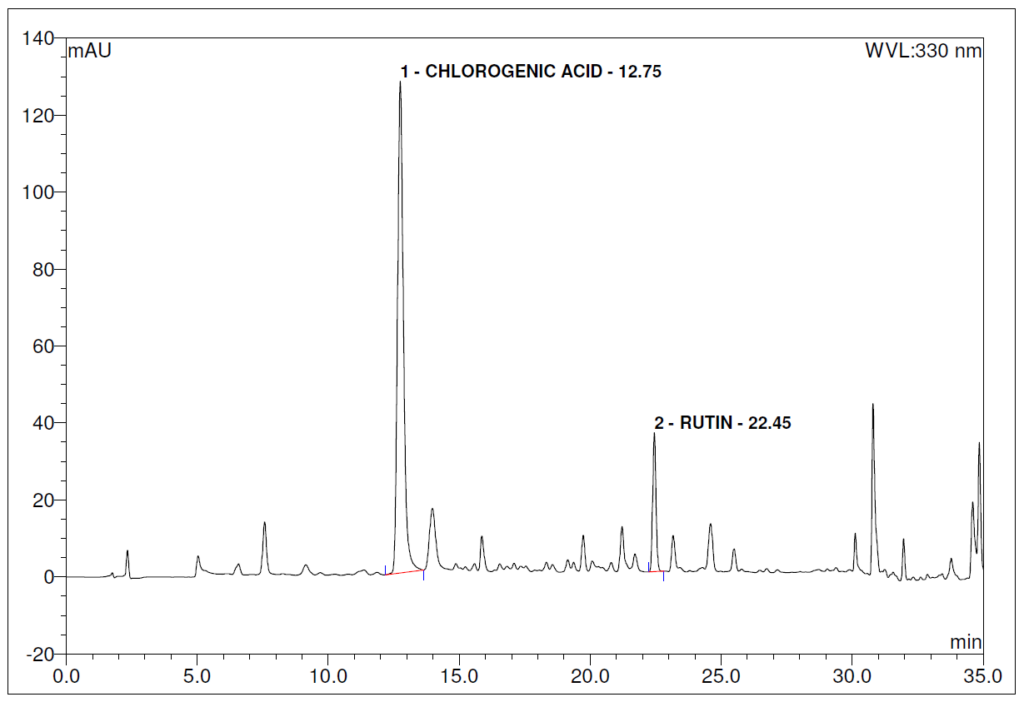
Figure 4 : Whole HPLC chromatogram of (a) chlorogenic acid standard solution (0.2 mg/mL) at tr = 12.80 min and rutin standard solution (0.2 mg/mL) at tr = 22.46 min and (b) ethanol (80%) extract of Morus alba dried leaf powder showing peaks at tr = 12.75 min corresponding to chlorogenic acid standard solution and tr = 22.45 min corresponding to rutin standard solution.
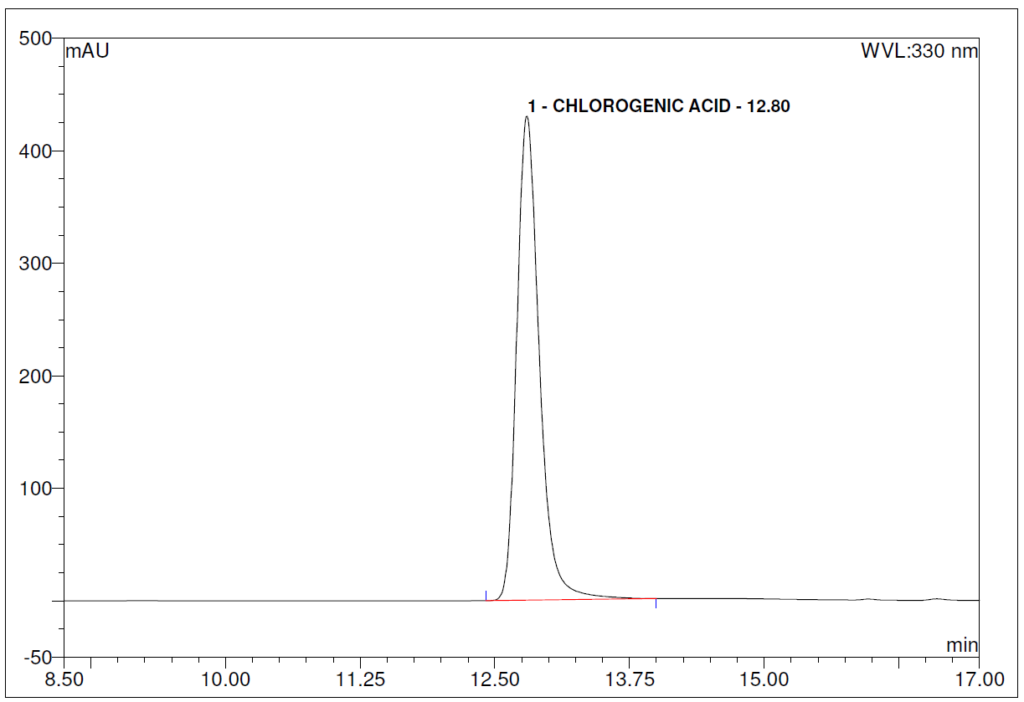

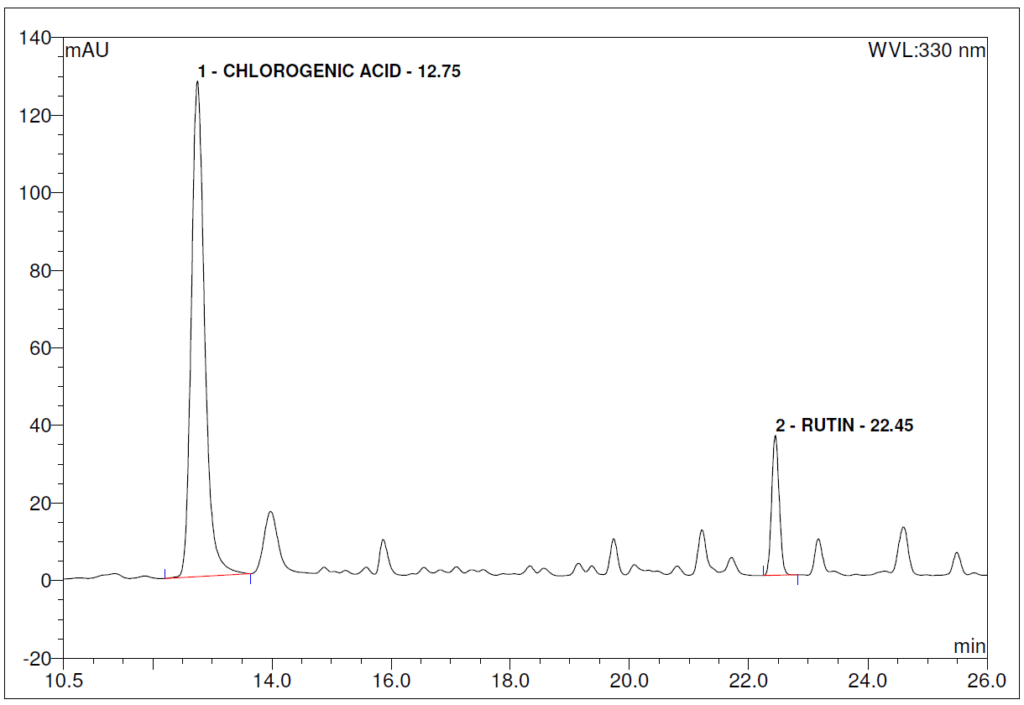
Figure 5 : HPLC chromatogram highlighting the elution region of (a) chlorogenic acid standard solution (0.2 mg/mL) at tr = 12.80 min and rutin standard solution (0.2 mg/mL) at tr = 22.46 min and (b) ethanol (80%) extract of Morus alba dried leaf powder showing peaks at tr = 12.75 min corresponding to chlorogenic acid standard solution and tr = 22.45 min corresponding to rutin standard solution.
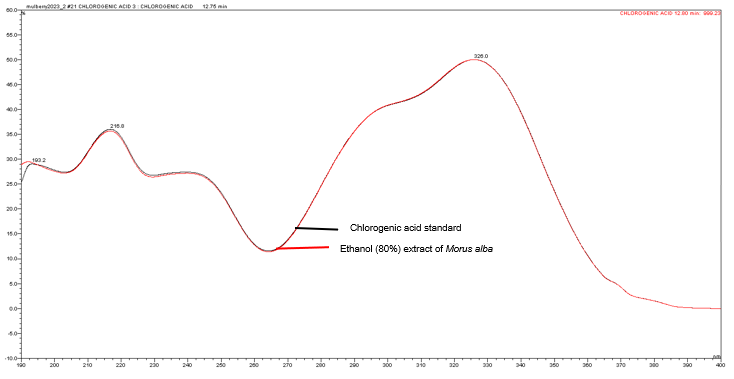
Figure 6 : UV spectra of chlorogenic acid standard solution (0.2 mg/mL) and ethanol (80%) extract of Morus alba dried leaf powder.
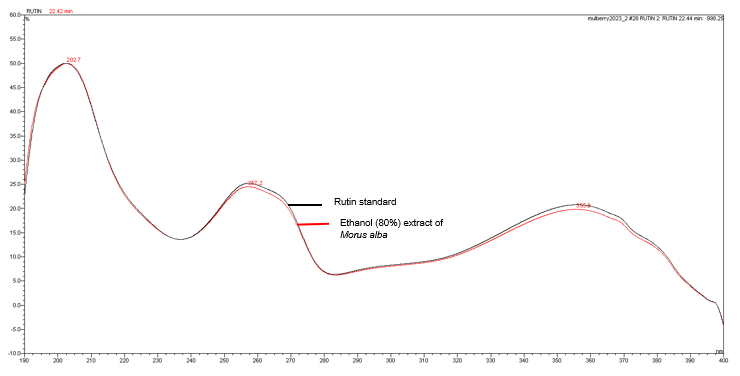
Figure 7 : UV spectra of rutin standard solution (0.2 mg/mL) and ethanol (80%) extract of Morus alba dried leaf powder.
Table 1: The relative retention time (RRT) of the peaks based on rutin as reference.
| Compound | RRT (min) |
| Chlorogenic acid | 0.57 |
| Rutin (as reference) | 1.00 |
Note: The RRTs listed above serve only as guidance
PURITY TESTS
| Foreign Matter |
| Not more than 2% |
| Ash Contents | |
| Total ash | Not more than 15% |
| Acid-insoluble ash | Not more than 4% |
| Water soluble ash | Not less than 7% |
| Loss on Drying |
| Not more than 9% |
| Extractive Values | |
|
For M. alba dried leaf powder of 0.355 mm size should be used. |
|
| Water-soluble extracts | |
| Hot method | Not less than 27% |
| Cold method | Not less than 18% |
| Ethanol-soluble extracts | |
| Hot method | Not less than 8% |
| Cold method | Not less than 3% |
SAFETY TESTS
| Heavy Metals | |
| Arsenic | Not more than 5.0 mg/kg |
| Mercury | Not more than 0.5 mg/kg |
| Lead | Not more than 10.0 mg/kg |
| Cadmium | Not more than 0.3 mg/kg |
| Microbial Limits | |
| Total bacterial count | Not more than 105 cfu/g |
| Total yeast and mould count | Not more than 104 cfu/g |
| Bile-tolerant gram negative | Not more than 104 cfu/g |
| Specific Pathogens | |
| Salmonella spp. | Absent in 25 g |
| Escherichia coli | Absent in 1 g |
| Staphylococcus aureus | Absent in 1 g |
| Pseudomonas aeruginosa | Absent in 1 g |
CHEMICAL CONSTITUENTS
Aqueous methanol extract (80%) of M. alba dried leaves was found to contain phenolic acid (e.g., chlorogenic acid) and flavonoid (e.g., rutin) [10].
Aqueous methanol extract (80%) of M. alba commercially available dietary tea leaves was found to contain flavonoids (e.g., rutin, isoquercetin and astragalin) [11].
Ethanol extracts (95% and 70%) of M. alba dried leaves were found to contain phenolic acid (e.g., chlorogenic acid) and flavonoids (e.g., rutin, quercitrin, kaempferol, and isoquercitrin) [12, 13].
Methanol extract of M. alba dried leaves were found to contain flavonoids (e.g., rutin) [7].
Hexane extract and supercritical carbon dioxide (CO2) extract of M. alba dried leaves were found to contain acylic diterpene (e.g., phytol), triterpenes (e.g., squalene, stigmasterol), triterpenoid (e.g., lupeol), vitamin E (e.g., alpha-tocopherol, beta-tocopherol, gamma-tocopherol, sigma-tocopherol), and sterols (e.g., beta-sitosterol, lanosterol, campesterol) [14].
MEDICINAL USES
Uses described in folk medicine, not supported by experimental or clinical data
Traditionally, the decoction of M. alba leaves is taken for detoxification and wound treatment. It is also used for the treatment of gonorrhea [15].
Biological and pharmacological activities supported by experimental data
Antioxidant activity
Ethanol extract (80%) of M. alba dried leaves exhibited 1,1-diphenyl-2-picrylhydrazyl (DPPH) radical scavenging activity with 50% inhibition concentration (IC50) value of 80.60 µg/mL compared to butylated hydroxyl anisole (BHA) standard (IC50 = 1.92 µg/mL) using DPPH assay [16].
Ethanol extract (80%) of M. alba dried leaves exhibited 2,2’-azinobis (3-ethylbenzothiazoline-6-sulfonic acid) diammonium salt (ABTS) radical scavenging activity with IC50 value of 65.27 µg/mL compared to trolox standard (IC50 = 5.98 µg/mL) using ABTS assay [16].
α-glucosidase inhibitory activity
Moracin D isolated from ethanol extract (70%) of M. alba dried leaves showed in vitro α-glucoside inhibitory activity with IC50 value of 2.54 µM compared to acarbose (IC50 = 181 µM) using α-glucosidase inhibitory assay [17].
Anti-tyrosinase activity
Epimeric mixture of (2R)/(2S)-7-methoxyl-8-ethyl-2’,4’-dihydroxylflavane-2’’-Ο-β-D-glucopyranoside isolated from ethanol extract (70%) of M. alba dried leaves showed in vitro tyrosinase inhibitory activity with IC50 value of 0.62 µM compared to kojic acid (IC50 = 15.9 µM) using tyrosinase inhibitory assay [17].
Cytotoxic activity
Moracin E and moracin M, both isolated from the 70% ethanol extract of Morus alba dried leaves, exhibited cytotoxicity against the lung cancer cell line A549. Moracin E demonstrated an IC50 value of 15.76 ± 1.22 µM, while moracin M exhibited an IC50 value of 18.85 ± 1.99 µM. In comparison, cisplatin, used as a reference, showed an IC50 value of 12.95 ± 1.31 µM in the cytotoxicity evaluation using the 3-(4,5)-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay [18].
Anti-proliferative activity
Purified leaf lectin from M. alba dried leaves showed anti-proliferative activity towards human breast cancer cell line (MCF-7) with 50% inhibition of cell growth (GI50) value of 8.5 µg/mL compared to cisplatin (GI50 value = 2 µg/mL) using MTT assay [19].
Purified lectin from M. alba dried leaves showed anti-proliferative activity towards colon cancer cell line (HCT-15) with GI50 value of 16 µg/mL compared to cisplatin (GI50 value = 1 µg/mL) using MTT assay [19].
Antidiabetic activity
Ethanol extract (50%) of M. alba dried leaves (500 mg/kg and 1000 mg/kg) administered orally to streptozotocin-induced diabetic male Sprague Dawley rats (200-250 g) daily for duration of 8 weeks decreased the blood glucose level (500 mg/kg; from 316 ± 44 mg/dL to 256 ± 24 mg/dL) and (1000 mg/kg; from 309 ± 54 mg/dL to 219 ± 22 mg/dL) compared to insulin (4 UI/kg; from 308 ± 90 to 208 ± 66 mg/dL) [20].
Clinical studies
A randomised, double blind, placebo-controlled trial involving 60 patients was conducted to determine the effects of M. alba leaf extract against metabolic profile in patients with type 2 diabetes mellitus. The treatment group (30 people) was given M. alba leaf extract (300 mg) twice a day for 12 weeks and were followed-up on 3-day record at baseline, 6 and 12 weeks of intervention. M. alba leaf extract intake significantly decreased insulin level from 12.7 to 11.7 mIU/L (p<0.026) and malondialdehyde level 2.9 to 2.7 µmol/L (p<0.001) from week 0 to week 12 respectively compared to placebo whereby insulin level from week 0 to week 12 was from 12.6 to 14.2 mIU/L and malondialdehyde level 2.6 to 3.3 µmol/L, respectively [21].
SAFETY INFORMATION
Preclinical studies (Toxicology studies)
14 days acute toxicity
Aqueous extract of M. alba dried leaves (7500 mg/kg body weight (BW)) administered orally twice a day to male and female Sprague Dawley rats (186.2-212.8 g). They were observed for 14 days. No mortality and no toxic effect were observed throughout the study. Lethal dose 50% (LD50) is more than 7500 mg/kg body weight [22].
Ethanol extract (70%) of M. alba dried leaves (300 mg/kg and 2000 mg/kg) administered orally to female Swiss mice (Mus musculus) (38-50 g) and were observed for 14 days. No death was found throughout the study period. However, based on hematological analysis there were significant (p<0.05) reductions in mean corpuscular volume (MCV) and mean corpuscular hemoglobin (MCHC) in animals treated with the extract at 2000 mg/kg compared to control group. All organs from animals administered with 2000mg/kg dose of extract showed alterations during necropsy. No observed adverse effect level (NOAEL) is less than 2000 mg/kg body weight [23].
30 days toxicity
Aqueous extract of M. alba dried leaves (1880 mg/kg BW, 3750 mg/kg BW, and 7500 mg/kg BW) was administered orally to weaning Sprague Dawley rats (male and female, 76-113 g) daily for 30 days. No mortality and no toxic effect were observed throughout the study. Lethal dose 50% (LD50) is more than 7500 mg/kg body weight [22].
Others (Adverse reactions, contraindications, side effects, warning, precautions)
Information and data have not been established.
DOSAGE
Information and data have not been established.
STORAGE
Store below 30°C. Protect from light and moisture.
REFERENCES
- World Flora Online [Internet]. Morus alba L. 2023 [cited 06 Jun 2023]. Available from: http://www.worldfloraonline.org/taxon/wfo-0000447905.
- Morus alba (mora) [Internet]. CABI. 2022. Available from: https://doi.org/10.1079/cabicompendium.34816.
- Nik Musa’adah Mustapha, Nik Zanariah Nik Mahmood, Nor Azah Mohd Ali, Haron N. Khazanah Perubatan Melayu. Tumbuhan Ubatan Jilid 12017. 260-1 p.
- Flowers of India 2016 [cited 2023 June]. Available from: http://www.flowersofindia.net.
- Huang HP, Ou TT, Wang CJ. Mulberry (sang shèn zǐ) and its bioactive compounds, the chemoprevention effects and molecular mechanisms in vitro and in vivo. Journal of traditional and complementary medicine. 2013;3(1):7-15.
- Commission CP. Pharmacopoeia of the People’s Republic of China. 2020.
- Ministry of Health and Welfare Taiwan RoC. Taiwan Herbal Pharmacopoeia 4th Edition English Version. 2022. p. 252-6.
- Plant Resources of South-East Asia No 12(1): Medicinal and poisonous plants 1 [Internet]. PROSEA Foundation, Bogor, Indonesia. 1999. Available from: prota4u.org/prosea.
- Flora of China [Internet]. Available from: http://www.efloras.org/florataxon.aspx?flora_id=2&taxon_id=200006379.
- Sánchez-Salcedo EM, Mena P, García-Viguera C, Hernández F, Martínez JJ. (Poly)phenolic compounds and antioxidant activity of white (Morus alba) and black (Morus nigra) mulberry leaves: Their potential for new products rich in phytochemicals. Journal of Functional Foods. 2015;18:1039-46.
- Gryn-Rynko A, Hołyńska-Iwan I, Janiak MA, Olszewska-Słonina D, Amarowicz R, Graczyk R. The impact of Morus alba L. leaf extract on intestinal ion transport. An in vitro study. Biomedicine & Pharmacotherapy. 2022;150:112939.
- Ji S, Zhu C, Gao S, Shao X, Chen X, Zhang H, et al. Morus alba leaves ethanol extract protects pancreatic islet cells against dysfunction and death by inducing autophagy in type 2 diabetes. Phytomedicine. 2021;83:153478.
- Oliveira AMd, Nascimento MFd, Ferreira MRA, Moura DFd, Souza TGdS, Silva GCd, et al. Evaluation of acute toxicity, genotoxicity and inhibitory effect on acute inflammation of an ethanol extract of Morus alba L. (Moraceae) in mice. Journal of Ethnopharmacology. 2016;194:162-8.
- Santos KA, Klein EJ, Fiorese ML, Palú F, da Silva C, da Silva EA. Extraction of Morus alba leaves using supercritical CO2 and ultrasound-assisted solvent: Evaluation of β-sitosterol content. The Journal of Supercritical Fluids. 2020;159:104752.
- I.H. Burkill MA, F.L.S. A dictionary of the economic products of the Malay Peninsula 1935.
- Eruygur N, Dural E. Determination of 1-Deoxynojirimycin by a developed and validated HPLC-FLD method and assessment of In-vitro antioxidant, α-Amylase and α-Glucosidase inhibitory activity in mulberry varieties from Turkey. Phytomedicine. 2019;53:234-42.
- Yang Z, Wang Y, Wang Y, Zhang Y. Bioassay-guided screening and isolation of α-glucosidase and tyrosinase inhibitors from leaves of Morus alba. Food Chemistry. 2012;131(2):617-25.
- Wu X, Li M, Wang X, Shen T, Wang S, Ren D. Two new 2-arylbenzofurnan derivatives from the leaves of Morus alba. Natural Product Research. 2019;33(2):204-11.
- Deepa M, Sureshkumar T, Satheeshkumar PK, Priya S. Purified mulberry leaf lectin (MLL) induces apoptosis and cell cycle arrest in human breast cancer and colon cancer cells. Chemico-Biological Interactions. 2012;200(1):38-44.
- Naowaboot J, Pannangpetch P, Kukongviriyapan V, Kukongviriyapan U, Nakmareong S, Itharat A. Mulberry leaf extract restores arterial pressure in streptozotocin-induced chronic diabetic rats. Nutrition Research. 2009;29(8):602-8.
- Taghizadeh M, Mohammad Zadeh A, Asemi Z, Farrokhnezhad AH, Memarzadeh MR, Banikazemi Z, et al. Morus alba leaf extract affects metabolic profiles, biomarkers inflammation and oxidative stress in patients with type 2 diabetes mellitus: A double-blind clinical trial. Clinical Nutrition ESPEN. 2022;49:68-73.
- Li Y, Zhang X, Liang C, Hu J, Yu Z. Safety evaluation of mulberry leaf extract: Acute, subacute toxicity and genotoxicity studies. Regulatory Toxicology and Pharmacology. 2018;95:220-6.
- de Oliveira AM, Mesquita MdS, da Silva GC, de Oliveira Lima E, de Medeiros PL, Paiva PMG, et al. Evaluation of Toxicity and Antimicrobial Activity of an Ethanolic Extract from Leaves of Morus alba L. (Moraceae). Evidence-Based Complementary and Alternative Medicine. 2015;2015:513978.










